
(a)
Interpretation: To write the molecular structure for the given compound.
Concept Introduction: Structural formulas are used to give the structures for the compounds. It helps in telling the exact number of atoms that are present in a compound along with
(a)
Answer to Problem 128A
The structural formula for heptane is represented as follows:
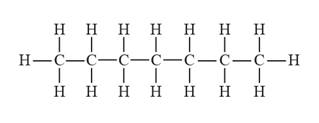
Explanation of Solution
With IUPAC names, it is easy to draw the structure of the compounds. Firstly, draw the hydrocarbon chain (parent chain), then identify the substituents groups or functional groups in the hydrocarbon name and attach them if present at proper positions. And lastly, add the hydrogen atoms wherever required.
In heptane, there is a long continuous carbon chain containing seven carbon atoms. Thus, the structural formula for heptane is given as follows:
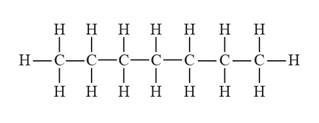
(b)
Interpretation: To write the molecular structure for the given compound.
Concept Introduction: Structural formulas are used to give the structures for the compounds. It helps in telling the exact number of atoms that are present in a compound along with functional groups if present.
(b)
Answer to Problem 128A
The structural formula for 2-phenylbutane is represented as follows:
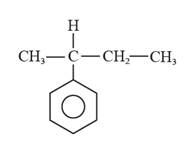
Explanation of Solution
With IUPAC names, it is easy to draw the structure of the compounds. Firstly, draw the hydrocarbon chain (parent chain), then identify the substituents groups or functional groups in the hydrocarbon name and attach them if present at proper positions. And lastly, add the hydrogen atoms wherever required.
In 2-phenylbutane, there is a phenyl ring i.e., a parent ring, and butane is substituted at the second carbon on this phenyl ring. Thus, the structural formula for 2-phenylbutane is given as follows:
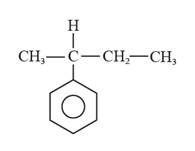
(c)
Interpretation: To write the molecular structure for the given compound.
Concept Introduction: Structural formulas are used to give the structures for the compounds. It helps in telling the exact number of atoms that are present in a compound along with functional groups if present.
(c)
Answer to Problem 128A
The structural formula for 2-methyl-3-hexene is represented as follows:
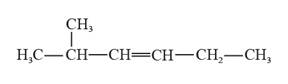
Explanation of Solution
With IUPAC names, it is easy to draw the structure of the compounds. Firstly, draw the hydrocarbon chain (parent chain), then identify the substituents groups or functional groups in the hydrocarbon name and attach them if present at proper positions. And lastly, add the hydrogen atoms wherever required.
In 2-methyl-3-hexene, the longest continuous chain is six carbon atoms chain that has a double bond at the third carbon. There is a methyl substituent that is present on the second carbon in this long continuous chain. Thus, the structural formula for 2-methyl-3-hexene is given as follows:
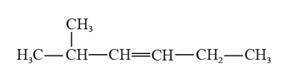
(d)
Interpretation: To write the molecular structure for the given compound.
Concept Introduction: Structural formulas are used to give the structures for the compounds. It helps in telling the exact number of atoms that are present in a compound along with functional groups if present.
(d)
Answer to Problem 128A
The structural formula for 1,3-diethylbenzene is represented as follows:
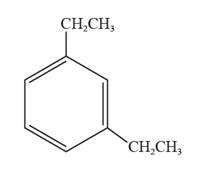
Explanation of Solution
With IUPAC names, it is easy to draw the structure of the compounds. Firstly, draw the hydrocarbon chain (parent chain), then identify the substituents groups or functional groups in the hydrocarbon name and attach them if present at proper positions. And lastly, add the hydrogen atoms wherever required.
In 1,3-diethylbenzene, the parent ring is benzene, and ethyl groups are substituted on the first and the third carbon atoms. Thus, the structural formula for 1,3-diethylbenzene is given as follows:
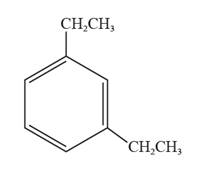
Chapter 24 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
- Explain what is the maximum absorbance of in which caffeine absorbs?arrow_forwardExplain reasons as to why the amount of caffeine extracted from both a singular extraction (5ml Mountain Dew) and a multiple extraction (2 x 5.0ml Mountain Dew) were severely high when compared to coca-cola?arrow_forwardProtecting Groups and Carbonyls 6) The synthesis generates allethrolone that exhibits high insect toxicity but low mammalian toxicity. They are used in pet shampoo, human lice shampoo, and industrial sprays for insects and mosquitos. Propose detailed mechanistic steps to generate the allethrolone label the different types of reagents (Grignard, acid/base protonation, acid/base deprotonation, reduction, oxidation, witting, aldol condensation, Robinson annulation, etc.) III + VI HS HS H+ CH,CH,Li III I II IV CI + P(Ph)3 V ༼ Hint: no strong base added VI S VII IX HO VIII -MgBr HgCl2,HgO HO. isomerization aqeuous solution H,SO, ༽༽༤༽༽ X MeOH Hint: enhances selectivity for reaction at the S X ☑arrow_forward
- Draw the complete mechanism for the acid-catalyzed hydration of this alkene. esc 田 Explanation Check 1 888 Q A slock Add/Remove step Q F4 F5 F6 A བྲA F7 $ % 5 @ 4 2 3 & 6 87 Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Ce W E R T Y U S D LL G H IK DD 요 F8 F9 F10 F1 * ( 8 9 0 O P J K L Z X C V B N M H He commandarrow_forwardExplanation Check F1 H₂O H₂ Pd 1) MCPBA 2) H3O+ 1) Hg(OAc)2, H₂O 2) NaBH4 OH CI OH OH OH hydration halohydrin formation addition halogenation hydrogenation inhalation hydrogenation hydration ☐ halohydrin formation addition halogenation formation chelation hydrogenation halohydrin formation substitution hydration halogenation addition Ohalohydrin formation subtraction halogenation addition hydrogenation hydration F2 80 F3 σ F4 F5 F6 1 ! 2 # 3 $ 4 % 05 Q W & Å © 2025 McGraw Hill LLC. All Rights Reserved. F7 F8 ( 6 7 8 9 LU E R T Y U A F9arrow_forwardShow the mechanism steps to obtain the lowerenergy intermediate: *see imagearrow_forward
- Soap is made by the previous reaction *see image. The main difference between one soap and another soap isthe length (number of carbons) of the carboxylic acid. However, if a soap irritates your skin, they mostlikely used too much lye.Detergents have the same chemical structure as soaps except for the functional group. Detergentshave sulfate (R-SO4H) and phosphate (R-PO4H2) functional groups. Draw the above carboxylic acidcarbon chain but as the two variants of detergents. *see imagearrow_forwardWhat are the reactions or reagents used? *see imagearrow_forwardWhat are the reactions or reagents used? *see imagearrow_forward
- Provide the mechanism for this transformation: *see imagearrow_forwardAssign all the signals individually (please assign the red, green and blue)arrow_forwardThe two pKa values of oxalic acid are 1.25 and 3.81. Why are they not the same value? Show the protontransfer as part of your explanation. *see imagearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





