
(a)
Interpretation: The formula of conjugate acid of base dimethylamide should be written.
Concept introduction: In accordance with Bronsted definition, an acid act as proton donor and a base can act as proton acceptor. Thus, in a typical acid-base reaction, the fundamental principle is a lone pair of base reaches out for an acidic proton. Similarly, curved arrows are used for departing conjugate base. After deprotonation, the species left with negative charge is refers as conjugate base of acid while the other with positive charge is termed conjugate acid of given base. For example:
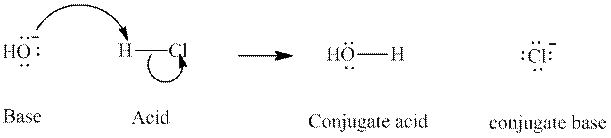
The strength of various conjugate acid-base pairs varies inversely to one another; the strong acid has weak conjugate base and strong base has weak conjugate acid and vice-versa.
(b)
Interpretation: The formula of conjugate acid of sulfide should be written.
Concept introduction: In accordance with Bronsted definition, an acid act as proton donor and a base can act as proton acceptor. Thus, in a typical acid-base reaction, the fundamental principle is a lone pair of base reaches out for an acidic proton. Similarly, curved arrows are used for departing conjugate base. After deprotonation, the species left with negative charge is refers as conjugate base of acid while the other with positive charge is termed conjugate acid of given base. For example:
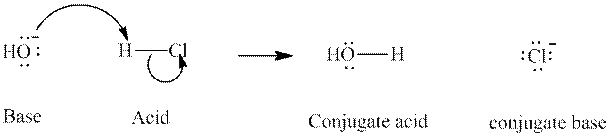
The strength of various conjugate acid-base pairs varies inversely to one another; the strong acid has weak conjugate base and strong base has weak conjugate acid and vice-versa.
(c)
Interpretation: The formula of conjugate acid of ammonia should be written.
Concept introduction: In accordance with Bronsted definition, an acid act as proton donor and a base can act as proton acceptor. Thus, in a typical acid-base reaction, the fundamental principle is a lone pair of base reaches out for an acidic proton. Similarly, curved arrows are used for departing conjugate base. After deprotonation, the species left with negative charge is refers as conjugate base of acid while the other with positive charge is termed conjugate acid of given base. For example:
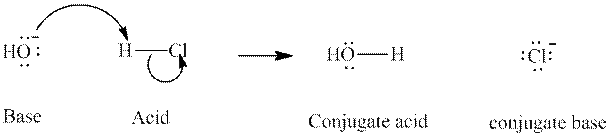
The strength of various conjugate acid-base pairs varies inversely to one another; the strong acid has weak conjugate base and strong base has weak conjugate acid and vice-versa.
(d)
Interpretation: The formula of conjugate acid of acetone should be written.
Concept introduction: In accordance with Bronsted definition, an acid act as proton donor and a base can act as proton acceptor. Thus, in a typical acid-base reaction, the fundamental principle is a lone pair of base reaches out for an acidic proton. Similarly, curved arrows are used for departing conjugate base. After deprotonation, the species left with negative charge is refers as conjugate base of acid while the other with positive charge is termed conjugate acid of given base. For example:
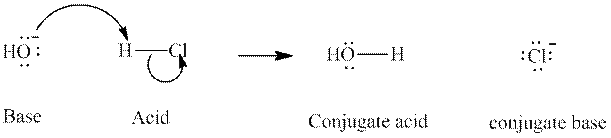
The strength of various conjugate acid-base pairs varies inversely to one another; the strong acid has weak conjugate base and strong base has weak conjugate acid and vice-versa.
(e)
Interpretation: The formula of conjugate acid of
Concept introduction: In accordance with Bronsted definition, an acid act as proton donor and a base can act as proton acceptor. Thus, in a typical acid-base reaction, the fundamental principle is a lone pair of base reaches out for an acidic proton. Similarly, curved arrows are used for departing conjugate base. After deprotonation, the species left with negative charge is refers as conjugate base of acid while the other with positive charge is termed conjugate acid of given base. For example:
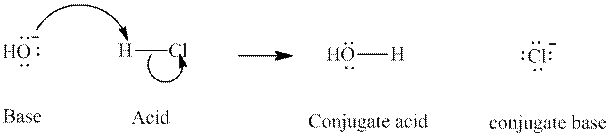
The strength of various conjugate acid-base pairs varies inversely to one another; the strong acid has weak conjugate base and strong base has weak conjugate acid and vice-versa.
Want to see the full answer?
Check out a sample textbook solution
Chapter 2 Solutions
EBK ORGANIC CHEMISTRY
- Please answer the questions in the photos and please revise any wrong answers. Thank youarrow_forward(Please be sure that 7 carbons are available in the structure )Based on the 1H NMR, 13C NMR, DEPT 135 NMR and DEPT 90 NMR, provide a reasoning step and arrive at the final structure of an unknown organic compound containing 7 carbons. Dept 135 shows peak to be positive at 128.62 and 13.63 Dept 135 shows peak to be negative at 130.28, 64.32, 30.62 and 19.10.arrow_forward-lease help me answer the questions in the photo.arrow_forward
- For the reaction below, the concentrations at equilibrium are [SO₂] = 0.50 M, [0] = 0.45 M, and [SO3] = 1.7 M. What is the value of the equilibrium constant, K? 2SO2(g) + O2(g) 2SO3(g) Report your answer using two significant figures. Provide your answer below:arrow_forwardI need help with this question. Step by step solution, please!arrow_forwardZn(OH)2(s) Zn(OH)+ Ksp = 3 X 10-16 B₁ = 1 x 104 Zn(OH)2(aq) B₂ = 2 x 1010 Zn(OH)3 ẞ3-8 x 1013 Zn(OH) B4-3 x 1015arrow_forward
- Help me understand this by showing step by step solution.arrow_forwardscratch paper, and the integrated rate table provided in class. our scratch work for this test. Content attribution 3/40 FEEDBACK QUESTION 3 - 4 POINTS Complete the equation that relates the rate of consumption of H+ and the rate of formation of Br2 for the given reaction. 5Br (aq) + BrO3 (aq) + 6H (aq) →3Br2(aq) + 3H2O(l) • Your answers should be whole numbers or fractions without any decimal places. Provide your answer below: Search 尚 5 fn 40 * 00 99+ 2 9 144 a [arrow_forward(a) Write down the structure of EDTA molecule and show the complex structure with Pb2+ . (b) When do you need to perform back titration? (c) Ni2+ can be analyzed by a back titration using standard Zn2+ at pH 5.5 with xylenol orange indicator. A solution containing 25.00 mL of Ni2+ in dilute HCl is treated with 25.00 mL of 0.05283 M Na2EDTA. The solution is neutralized with NaOH, and the pH is adjusted to 5.5 with acetate buffer. The solution turns yellow when a few drops of indicator are added. Titration with 0.02299 M Zn2+ requires 17.61 mL to reach the red end point. What is the molarity of Ni2+ in the unknown?arrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

