
Concept explainers
(a)
Interpretation: The process with proper usage of curved arrows and the ones with incorrect usage should be indicated and latter cases should be corrected.
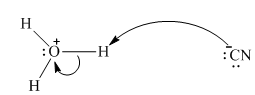
Concept introduction: The fundamental electrostatics suggests that electrons have more affinity for electron deficient sites in an organic compound or positive charge. The curved arrow is appropriate mechanism to depict electron movement that occurs from electron rich species to electron-deficient species.
(b)
Interpretation:The process with proper usage of curved arrows and the ones with incorrect usage should be indicated and latter cases should be corrected.
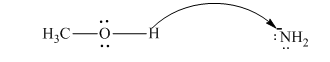
Concept introduction:The fundamental electrostatics suggests that electrons have more affinity for electron deficient sites in an organic compound or positive charge. The curved arrow is appropriate mechanism to depict electron movement that occurs from electron rich species to electron-deficient species.
(c)
Interpretation:The process with proper usage of curved arrows and the ones with incorrect usage should be indicated and latter cases should be corrected.
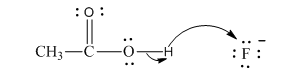
Concept introduction:The fundamental electrostatics suggests that electrons have more affinity for electron deficient sites in an organic compound or positive charge. The curved arrow is appropriate mechanism to depict electron movement that occurs from electron rich species to electron-deficient species.
(d)
Interpretation:The process with proper usage of curved arrows and the ones with incorrect usage should be indicated and latter cases should be corrected.
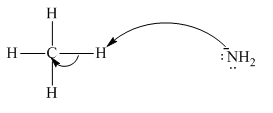
Concept introduction: The fundamental electrostatics suggests that electrons have more affinity for electron deficient sites in an organic compound. or positive charge. The curved arrow is appropriate mechanism to depict electron movement that occurs from electron rich species to electron deficient species.
(e)
Interpretation:The process with proper usage of curved arrows and the ones with incorrect usage should be indicated and latter cases should be corrected.
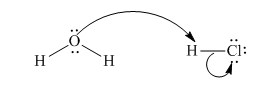
Concept introduction: The fundamental electrostatics suggests that electrons have more affinity for electron deficient sites in an organic compound. or positive charge. The curved arrow is appropriate mechanism to depict electron movement that occurs from electron rich species to electron deficient species.
(f)
Interpretation:The process with proper usage of curved arrows and the ones with incorrect usage should be indicated and latter cases should be corrected.
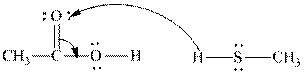
Concept introduction: The fundamental electrostatics suggests that electrons have more affinity for electron deficient sites in an organic compound. or positive charge. The curved arrow is appropriate mechanism to depict electron movement that occurs from electron rich species to electron deficient species.
Bimolecular substitution or
Want to see the full answer?
Check out a sample textbook solution
Chapter 2 Solutions
EBK ORGANIC CHEMISTRY
- Draw the major product of this reaction. Nitropropane reacts + pent-3-en-2-one reacts with NaOCH2CH3, CH3CHOHarrow_forwardIndicate whether the product formed in the reaction exhibits tautomerism. If so, draw the structure of the tautomers. OC2H5 + CoHs-NH-NH,arrow_forwardExplain how substitutions at the 5-position of barbituric acid increase the compound's lipophilicity.arrow_forward
- Explain how substitutions at the 5-position of phenobarbital increase the compound's lipophilicity.arrow_forwardName an interesting derivative of barbituric acid, describing its structure.arrow_forwardBriefly describe the synthesis mechanism of barbituric acid from the condensation of urea with a β-diketone.arrow_forward
- Given the hydrazones indicated, draw the structures of the enamines that can be formed. Indicate the most stable enamine (explain). C6H5 C6H5 H C6H5 Harrow_forward4. Propose a Synthesis for the molecule below. You may use any starting materials containing 6 carbons or less (reagents that aren't incorporated into the final molecule such as PhзP do not count towards this total, and the starting material can have whatever non-carbon functional groups you want), and any of the reactions you have learned so far in organic chemistry I, II, and III. Your final answer should show each step separately, with intermediates and conditions clearly drawn.arrow_forwardIndicate the importance of the indole ring. Find a representative example and list 5 structures.arrow_forward
- ΌΗ 1) V2 CO 3 or Nalt In منهarrow_forward6. The equilibrium constant for the reaction 2 HBr (g) → H2(g) + Br2(g) Can be expressed by the empirical formula 11790 K In K-6.375 + 0.6415 In(T K-¹) - T Use this formula to determine A,H as a function of temperature. Calculate A,-H at 25 °C and at 100 °C.arrow_forward3. Nitrosyl chloride, NOCI, decomposes according to 2 NOCI (g) → 2 NO(g) + Cl2(g) Assuming that we start with no moles of NOCl (g) and no NO(g) or Cl2(g), derive an expression for Kp in terms of the equilibrium value of the extent of reaction, Seq, and the pressure, P. Given that K₂ = 2.00 × 10-4, calculate Seq/ of 29/no when P = 0.080 bar. What is the new value по ƒª/ at equilibrium when P = 0.160 bar? Is this result in accord with Le Châtelier's Principle?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning



