
Concept explainers
(a)
Interpretation:
The empirical formula of the given acid has to be determined.
Concept introduction:
Maleic acid is prepared by the catalytic oxidation of benzene.It is a dicarboxylic acid ,that is, it has two
It is cis– isomer of butenedioic acid.Molecular formula is
The structure of maleic acid is as shown below.

Empirical formula of a compound is the smallest integer ratio of numbers of each element presented in that compound.
Molecular formula of a compound is integer multiple of empirical formula, the integer is depend upon the mass of empirical formula and the molecular mass of the compound.
The number of moles of any substance can be determined using the equation,
(a)
Answer to Problem 113SCQ
Empirical formula of the acid is
Explanation of Solution
Assuming the molecular formula of the compound is
It dissociates into carbondioxide and water. The equation is as follows.
Let’s calculate the moles of C in
Let’s calculate the moles of
Weight of Hydrogen =
Let’s calculate the weight of Oxygen:
Let’s calculate the mole of Oxygen:
Let’s calculate the mole ratio of each element:
Therefore, the empirical formula of the given compound is
(b)
Interpretation:
The molecular formula of the given acid has to be determined.
Concept introduction:
Maleic acid is prepared by the catalytic oxidation of benzene.It is a dicarboxylic acid ,that is, it has two carboxylic acid groups.
It is cis– isomer of butenedioic acid.Molecular formula is
The structure of maleic acid is as shown below.

Empirical formula of a compound is the smallest integer ratio of numbers of each element presented in that compound.
Molecular formula of a compound is integer multiple of empirical formula, the integer is depend upon the mass of empirical formula and the molecular mass of the compound.
The number of moles of any substance can be determined using the equation,
(b)
Answer to Problem 113SCQ
Molecular formula of the acid is
Explanation of Solution
According to the law of gram equivalents, equivalent acid is equal to the equivalents of base.
Let’s calculate the molecular formula:
Substitute the ‘n’ value we get molecular formula of acid.
(c)
Interpretation:
The Lewis structure of the given acid has to be drawn.
Concept introduction:
Maleic acid is prepared by the catalytic oxidation of benzene.It is a dicarboxylic acid ,that is, it has two carboxylic acid groups.
It is cis– isomer of butenedioic acid.Molecular formula is
The structure of maleic acid is as shown below.

Lewis structures are diagrams that represent the
It is also known as Lewis dot structures which represent the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
(c)
Explanation of Solution
The Lewis structure indicates the all unpaired electrons present in the atom in the molecules.
The Lewis structure of Maleic is as follows.
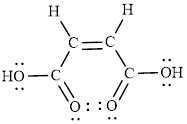
(d)
Interpretation:
The hybridization used by the carbon atom in the given acid compound has to be described.
Concept introduction:
Maleic acid is prepared by the catalytic oxidation of benzene.It is a dicarboxylic acid ,that is, it has two carboxylic acid groups.
It is cis– isomer of butenedioic acid.Molecular formula is
The structure of maleic acid is as shown below.

Hybridization is the mixing of valence atomic orbitals to get equivalent hybridized orbitals that having similar characteristics and energy.
(d)
Explanation of Solution
The Lewis structure indicates the all unpaired electrons present in the atom in the molecules.
The Lewis structure of Maleic is as follows.

The geometry around the entire carbon atoms in the molecule is trigonal planar, which means these carbon atoms used
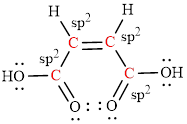
(e)
Interpretation:
The bond angles around each C-atom in the given molecule has to be identified.
Concept introduction:
Maleic acid is prepared by the catalytic oxidation of benzene.It is a dicarboxylic acid ,that is, it has two carboxylic acid groups.
It is cis– isomer of butenedioic acid.Molecular formula is
The structure of maleic acid is as shown below.

Hybridization is the mixing of valence atomic orbitals to get equivalent hybridized orbitals that having similar characteristics and energy.
(e)
Explanation of Solution
The Lewis structure of Maleic is as follows.

The geometry around the entire carbon atoms in the molecule is trigonal planar, which means these carbon atoms used

The geometry around each carbon atom in the given compound is trigonal planar and so the bond angle is
Want to see more full solutions like this?
Chapter 23 Solutions
Chemistry & Chemical Reactivity, Hybrid Edition (with OWLv2 24-Months Printed Access Card)
- Calculate the ionization energy of He+ and Li²+ ions in their ground states. Thannnxxxxx sirrr Ahehehehehejh27278283-4;*; shebehebbw $+$;$-;$-28283773838 hahhehdvaarrow_forwardPlleeaasseee solllveeee question 3 andd thankss sirr, don't solve it by AI plleeaasseee don't use AIarrow_forwardCalculate the chemical shifts in 13C and 1H NMR for 4-chloropropiophenone ? Write structure and label hydrogens and carbonsarrow_forward
- 4. Read paragraph 4.15 from your textbook, use your calculated lattice energy values for CuO, CuCO3 and Cu(OH)2 an explain thermal decomposition reaction of malachite: Cu2CO3(OH)2 →2CuO + H2O + CO2 (3 points)arrow_forwardPlease sirrr soollveee these parts pleaseeee and thank youuuuuarrow_forwardIII O Organic Chemistry Using wedges and dashes in skeletal structures Draw a skeletal ("line") structure for each of the molecules below. Be sure your structures show the important difference between the molecules. key O O O O O CHON Cl jiii iiiiiiii You can drag the slider to rotate the molecules. Explanation Check Click and drag to start drawing a structure. Q Search X G ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use F 3 W C 3/5arrow_forward
- 3. Use Kapustinskii's equation and data from Table 4.10 in your textbook to calculate lattice energies of Cu(OH)2 and CuCO3 (4 points)arrow_forward2. Copper (II) oxide crystalizes in monoclinic unit cell (included below; blue spheres 2+ represent Cu²+, red - O²-). Use Kapustinski's equation (4.5) to calculate lattice energy for CuO. You will need some data from Resource section of your textbook (p.901). (4 points) CuOarrow_forwardWhat is the IUPAC name of the following compound? OH (2S, 4R)-4-chloropentan-2-ol O (2R, 4R)-4-chloropentan-2-ol O (2R, 4S)-4-chloropentan-2-ol O(2S, 4S)-4-chloropentan-2-olarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





