
Concept explainers
(a)
Interpretation: For the given
Concept introduction:
Amines are the hydrocabons which have nitrogen
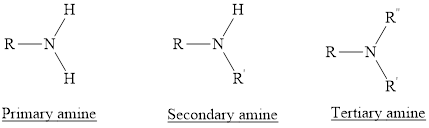
(1) Primary Amines: The nitrogen atoms is substituted by one alkyl group and two hydrogen atoms are also attached to nitrogen atom is called as primary amine. For example,
(2) Secondary Amines: The nitrogen atoms is substituted by two alkyl groups and one hydrogen atom is also attached to nitrogen atom is called as secondary amine.
(3) Tertiary Amines: The nitrogen atoms is substituted by three alkyl groups and no hydrogen atom is attached to nitrogen atom is called as tertiary amine.
(a)
Answer to Problem 39PS
The molecular formula of the given amine is
Explanation of Solution
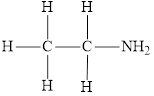
The molecular formula of the given amine can be written by using its given name ethylamine.
The ethyl group
Therefore molecular formula will have on ethyl group and two hydrogen atoms attached to nitrogen atom, that is
The structural formula for this amine is drawn as follows,

(b)
Interpretation: For the given amines structural formula has to be drawn and also their molecular formula has to be given.
Concept introduction:
Amines are the hydrocabons which have nitrogen
(1) Primary Amines: The nitrogen atoms is substituted by one alkyl group and two hydrogen atoms are also attached to nitrogen atom is called as primary amine. For example,
(2) Secondary Amines: The nitrogen atoms is substituted by two alkyl groups and one hydrogen atom is also attached to nitrogen atom is called as secondary amine.
(3) Tertiary Amines: The nitrogen atoms is substituted by three alkyl groups and no hydrogen atom is attached to nitrogen atom is called as tertiary amine.

(b)
Answer to Problem 39PS
The molecular formula of the given amine is
Explanation of Solution
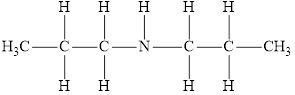
The molecular formula of the given amine can be written by using its given name dipropylamine.
The two propyl groups
It is secondary amine as two alkyl groups are attached to nitrogen atom.
Therefore molecular formula will have two propyl groups and one hydrogen atom attached to nitrogen atom, that is
The structural formula for this amine is drawn as follows,

(c)
Interpretation: For the given amines structural formula has to be drawn and also their molecular formula has to be given.
Concept introduction:
Amines are the hydrocabons which have nitrogen
(1) Primary Amines: The nitrogen atoms is substituted by one alkyl group and two hydrogen atoms are also attached to nitrogen atom is called as primary amine. For example,
(2) Secondary Amines: The nitrogen atoms is substituted by two alkyl groups and one hydrogen atom is also attached to nitrogen atom is called as secondary amine.
(3) Tertiary Amines: The nitrogen atoms is substituted by three alkyl groups and no hydrogen atom is attached to nitrogen atom is called as tertiary amine.

(c)
Answer to Problem 39PS
The molecular formula of the given amine is
Explanation of Solution
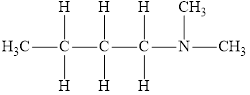
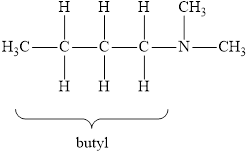
The molecular formula of the given amine can be written by using its given name butyl dimethylamine.
The two methyl groups
It is tertiary amine as three alkyl groups are attached to nitrogen atom.
Therefore molecular formula will have two methyl groups, one butyl group and no hydrogen atom attached to nitrogen atom, that is
The structural formula for this amine is drawn as follows,
(d)
Interpretation: For the given amines structural formula has to be drawn and also their molecular formula has to be given.
Concept introduction:
Amines are the hydrocabons which have nitrogen
(1) Primary Amines: The nitrogen atoms is substituted by one alkyl group and two hydrogen atoms are also attached to nitrogen atom is called as primary amine. For example,
(2) Secondary Amines: The nitrogen atoms is substituted by two alkyl groups and one hydrogen atom is also attached to nitrogen atom is called as secondary amine.
(3) Tertiary Amines: The nitrogen atoms is substituted by three alkyl groups and no hydrogen atom is attached to nitrogen atom is called as tertiary amine.

(d)
Answer to Problem 39PS
The molecular formula of the given amine is
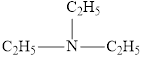
Explanation of Solution
The molecular formula of the given amine can be written by using its given name triethylamine.
The three ethyl groups
It is tertiary amine as three alkyl groups are attached to nitrogen atom.
Therefore molecular formula will have three ethyl groups, no hydrogen atom attached to nitrogen atom, that is
Want to see more full solutions like this?
Chapter 23 Solutions
Chemistry & Chemical Reactivity, Hybrid Edition (with OWLv2 24-Months Printed Access Card)
- Can you help me understand the CBC method on metal bridging by looking at this problem?arrow_forwardA partir de Aluminio y Co(NO3)2ꞏ6H2O, indicar las reacciones a realizar para obtener Azul de Thenard (Al2CoO4).arrow_forwardTo obtain Thenard Blue (Al2CoO4), the following reaction is correct (performed in an oven):Al(OH)3 + Co(OH)2 → Al2CoO4 + 4 H2Oarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
