
Concept explainers
(a)
Interpretation:
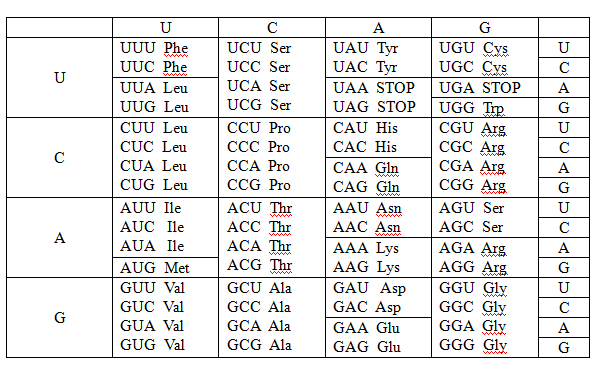
The anticodon and the amino acid of CGG codon should be written.
Concept Introduction:
Anticodon is the tRNA sequence of codon. Each triplet of
(b)
Interpretation:
The anticodon and the amino acid of GGG codon should be written.
Concept Introduction:
Anticodon is the tRNA sequence of codon. Each triplet of nucleic acid represents a single amino acid.

(c)
Interpretation:
The anticodon and the amino acid of UCC codon should be written.
Concept Introduction:
Anticodon is the tRNA sequence of codon. Each triplet of nucleic acid represent a single amino acid.

(d)
Interpretation:
The anticodon and the amino acid of AUA codon should be written.
Concept Introduction:
Anticodon is the tRNA sequence of codon. Each triplet of nucleic acid represent a single amino acid.

(e)
Interpretation:
The anticodon and the amino acid of CCU codon should be written.
Concept Introduction:
Anticodon is the tRNA sequence of codon. Each triplet of nucleic acid represents a single amino acid.

(f)
Interpretation:
The anticodon and the amino acid of GCC codon should be written.
Concept Introduction:
Anticodon is the tRNA sequence of codon. Each triplet of nucleic acid represents a single amino acid.

Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
General, Organic, & Biological Chemistry
- Some of the theories used to describe interface structure can be distinguished by:1. the measured potential difference.2. the distribution of ions in solution.3. the calculation of charge density.4. the external Helmoltz plane.arrow_forwardWhen talking about the acidity of carboxylic acids, is it the same thing to say higher or stronger acidity?arrow_forwardUsing the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$arrow_forward
- Indicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forwardIndicate the formula of the product obtained by reacting D-Galactose with hydroxylamine.arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning





