
(a)
Interpretation: The difference in basicity between ortho- or para- and meta-nitroanilines has to be discussed
Concept Introduction:
Resonance is a mental exercise and method within the
To find: para-nitroaniline is an order of magnitude less basic than meta-nitroaniline. Find the reason for the given statement
Find the delocalization behavior of para-nitroaniline
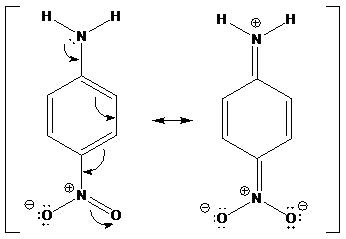
(b)
Interpretation: The difference in basicity between ortho- or para- and meta-nitroanilines has to be discussed
Concept Introduction:
Resonance is a mental exercise and method within the Valence Bond Theory of bonding that describes the delocalization of electrons within molecules. The net sum of valid resonance structures is defined as a resonance hybrid, which represents the overall delocalization of electrons within the molecule. A molecule that has several resonance structures is more stable than one with fewer. If the delocalization of electrons within molecules is more, the compound is less basic and vice versa.
To find: Finding the basicity of ortho-nitroaniline which is closer in value to meta- or to para-nitroaniline
Find the delocalization behavior of ortho-nitroaniline

Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
- The compound in the figure is reacted with 10 n-butyllihium, 2° propanone, and 3º H2O. Draw and name the products obtained. SiMe3arrow_forwardCaffeine (C8H10N4O2, pictured below) is a weak base. The pKb of caffeine is 10.4. What is the pH of a 0.0155 M solution of caffeine?arrow_forward2-Cyclopentyl-2-methyl-1,3-dioxolane is reacted with H₂SO₄. Draw and name the structures of the products.arrow_forward
- Indicate the products of the reaction of 1-cyclohexyl-2,2-dimethylpropan-1-one with CH3CO3H (). Draw the structures of the compounds.arrow_forwardWrite chemical equations for: the reaction of benzoic acid chloride with grignard reagent [CH3MgX] the reaction of butanoic acid with methyl amine [CH3NH2]arrow_forward2-(3-Aminopropyl)cyclohexan-1-one is reacted with H₂SO₄. Draw the structures of the products.arrow_forward
- Please help me solve number 2arrow_forwardChoose the best reagents to complete the following reaction. 오 Na2Cr2O7 H2SO4, H2O Problem 22 of 35 A Na2Cr2O7 H2SO4, H2O H2/Pt B pressure OH 1. NaBH4 C 2. H3O+ D DMP (Dess-Martin Periodinane) CH2Cl2 CrO3 Done Dramabana_Minor Submitarrow_forwardIndicate the products of the reaction of Cycloheptanone with pyrrolidine (cat. H+). Draw the structures of the compounds.arrow_forward
- Indicate the products of the reaction of 2-(3-aminopropyl)cyclohexan-1-one with H2SO4. Draw the structures of the compounds.arrow_forwardIndicate the products of the reaction of 2-cyclopentyl-2-methyl-1,3-dioxolane with H3O+. Draw the structures of the compounds.arrow_forwardQuestion 4 For the molecule shown below, (7 marks): A) Sketch the Newman projection for the view looking along the bond from the perspective of the arrow. B) Then, draw the Newman projection for each 60° rotation along the bond until it returns to the starting point. C) Clearly indicate which Newman projection is the one we see in the structure shown below, and clearly indicate which Newman projection is the highest in energy and which is the lowest in energy. H H Me 'H Me Mearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





