
(a)
Interpretation: Hexylamines have to be synthesized from various starting compounds.
Concept Introduction: The general formula for hexylamine is C6H13NH2. There are several methods available to prepare primary
Step-1: Formation of potassium phthalimide (deprotonation)
Potassium phthalimide in alkaline KOH acts as the reagent which has negatively charged phthalimide. It is formed by the reaction between phthalimide and potassium hydroxide.
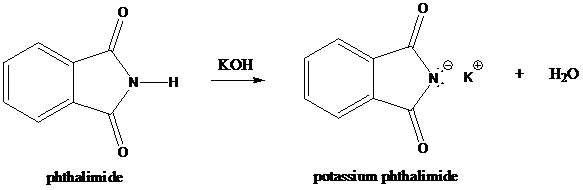
Step-2: Formation of R−N bond by SN2 nucleophilic substitution
The negative charged nitrogen atom in phthalimide can easily attract the positive side of R−X. In primary
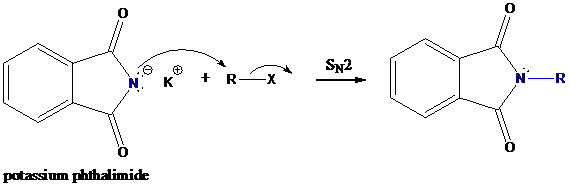
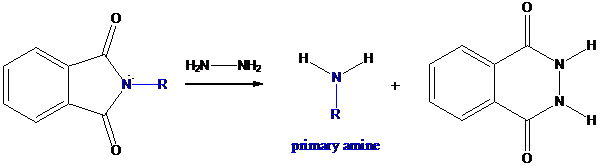
Step-3: Formation of primary amine by hydrolysis
The resultant product further goes for hydrolysis using hydrazine as the reagent. This reaction also follows nucleophilic substitution reaction. Finally, primary amine is formed with a side product of hydrazine derivative.
(b)
Interpretation: Hexylamines have to be synthesized from various starting compounds.
Concept Introduction: The general formula for hexylamine is C6H13NH2. There are several methods available to prepare primary amines. Among them, Gabriel synthesis plays a very important role for preparing it. In this method, secondary and tertiary amines are not formed as side products. It involves in three steps.
Step-1: Formation of potassium phthalimide (deprotonation)
Potassium phthalimide in alkaline KOH acts as the reagent which has negatively charged phthalimide. It is formed by the reaction between phthalimide and potassium hydroxide.
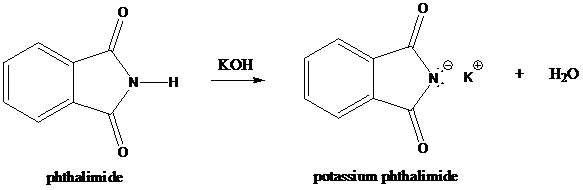
Step-2: Formation of R−N bond by SN2 nucleophilic substitution
The negative charged nitrogen atom in phthalimide can easily attract the positive side of R−X. In primary alkyl halides (R−X), R and X get positive and negative charges, respectively when they ionize. As a result, a bond between nitrogen of phthalimide and carbon of R is formed. This is SN2 nucleophilic substitution reaction. Halogen atom is going away as halide anion.
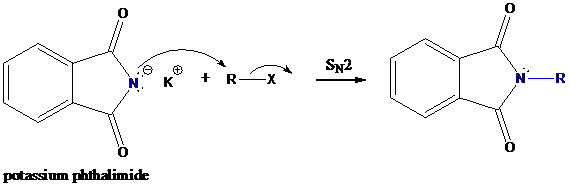
Step-3: Formation of primary amine by hydrolysis
The resultant product further goes for hydrolysis using hydrazine as the reagent. This reaction also follows nucleophilic substitution reaction. Finally, primary amine is formed with a side product of hydrazine derivative.

(c)
Interpretation: Hexylamines have to be synthesized from various starting compounds.
Concept Introduction: The general formula for hexylamine is C6H13NH2. There are several methods available to prepare primary amines. Among them, Gabriel synthesis plays a very important role for preparing it. In this method, secondary and tertiary amines are not formed as side products. It involves in three steps.
Step-1: Formation of potassium phthalimide (deprotonation)
Potassium phthalimide in alkaline KOH acts as the reagent which has negatively charged phthalimide. It is formed by the reaction between phthalimide and potassium hydroxide.
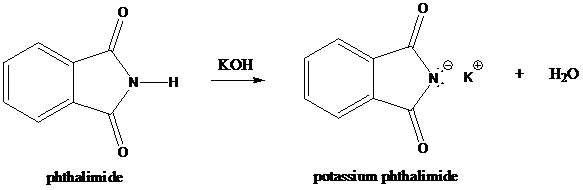
Step-2: Formation of R−N bond by SN2 nucleophilic substitution
The negative charged nitrogen atom in phthalimide can easily attract the positive side of R−X. In primary alkyl halides (R−X), R and X get positive and negative charges, respectively when they ionize. As a result, a bond between nitrogen of phthalimide and carbon of R is formed. This is SN2 nucleophilic substitution reaction. Halogen atom is going away as halide anion.
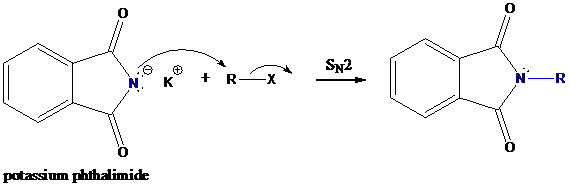
Step-3: Formation of primary amine by hydrolysis
The resultant product further goes for hydrolysis using hydrazine as the reagent. This reaction also follows nucleophilic substitution reaction. Finally, primary amine is formed with a side product of hydrazine derivative.

(d)
Interpretation: Hexylamines have to be synthesized from various starting compounds.
Concept Introduction: The general formula for hexylamine is C6H13NH2. There are several methods available to prepare primary amines. Among them, Gabriel synthesis plays a very important role for preparing it. In this method, secondary and tertiary amines are not formed as side products. It involves in three steps.
Step-1: Formation of potassium phthalimide (deprotonation)
Potassium phthalimide in alkaline KOH acts as the reagent which has negatively charged phthalimide. It is formed by the reaction between phthalimide and potassium hydroxide.
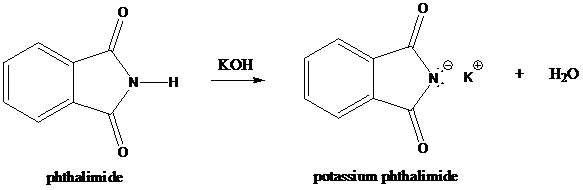
Step-2: Formation of R−N bond by SN2 nucleophilic substitution
The negative charged nitrogen atom in phthalimide can easily attract the positive side of R−X. In primary alkyl halides (R−X), R and X get positive and negative charges, respectively when they ionize. As a result, a bond between nitrogen of phthalimide and carbon of R is formed. This is SN2 nucleophilic substitution reaction. Halogen atom is going away as halide anion.
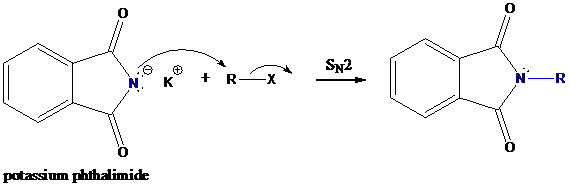
Step-3: Formation of primary amine by hydrolysis
The resultant product further goes for hydrolysis using hydrazine as the reagent. This reaction also follows nucleophilic substitution reaction. Finally, primary amine is formed with a side product of hydrazine derivative.

Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
- Including activity, calculate the solubility of Pb(IO3)2 in a matrix of 0.020 M Mg(NO3)2.arrow_forwardIncluding activity coefficients, find [Hg22+] in saturated Hg2Br2 in 0.00100 M KBr.arrow_forwardIncluding activity, calculate the pH of a 0.010 M HCl solution with an ionic strength of 0.10 M.arrow_forward
- Can I please get the graph 1: Concentration vs. Density?arrow_forwardOrder the following series of compounds from highest to lowest reactivity to electrophilic aromatic substitution, explaining your answer: 2-nitrophenol, p-Toluidine, N-(4-methylphenyl)acetamide, 4-methylbenzonitrile, 4-(trifluoromethyl)benzonitrile.arrow_forwardOrdene la siguiente serie de compuestos de mayor a menor reactividad a la sustitución aromática electrofílica, explicando su respuesta: ácido bencenosulfónico, fluorobenceno, etilbenceno, clorobenceno, terc-butilbenceno, acetofenona.arrow_forward
- Can I please get all final concentrations please!arrow_forwardState the detailed mechanism of the reaction of benzene with isopropanol in sulfuric acid.arrow_forwardDo not apply the calculations, based on the approximation of the stationary state, to make them perform correctly. Basta discard the 3 responses that you encounter that are obviously erroneous if you apply the formula to determine the speed of a reaction. For the decomposition reaction of N2O5(g): 2 N2O5(g) · 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 -> NO2 + NO3_(K1) NO2 + NO3 →> N2O5 (k-1) → NO2 + NO3 → NO2 + O2 + NO (K2) NO + N2O5 → NO2 + NO2 + NO2 (K3) Give the expression for the acceptable rate. (A). d[N₂O] dt = -1 2k,k₂[N205] k₁+k₂ d[N₂O5] (B). dt =-k₁[N₂O₂] + k₁[NO2][NO3] - k₂[NO2]³ (C). d[N₂O] dt =-k₁[N₂O] + k₁[N205] - K3 [NO] [N205] (D). d[N2O5] =-k₁[NO] - K3[NO] [N₂05] dtarrow_forward
- A 0.10 M solution of acetic acid (CH3COOH, Ka = 1.8 x 10^-5) is titrated with a 0.0250 M solution of magnesium hydroxide (Mg(OH)2). If 10.0 mL of the acid solution is titrated with 20.0 mL of the base solution, what is the pH of the resulting solution?arrow_forwardFor the decomposition reaction of N2O5(g): 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 NO2 + NO3 (K1) | NO2 + NO3 → N2O5 (k-1) | NO2 + NO3 NO2 + O2 + NO (k2) | NO + N2O51 NO2 + NO2 + NO2 (K3) → Give the expression for the acceptable rate. → → (A). d[N205] dt == 2k,k₂[N₂O₂] k₁+k₁₂ (B). d[N2O5] =-k₁[N₂O] + k₁[NO₂] [NO3] - k₂[NO₂]³ dt (C). d[N2O5] =-k₁[N₂O] + k [NO] - k₂[NO] [NO] d[N2O5] (D). = dt = -k₁[N2O5] - k¸[NO][N₂05] dt Do not apply the calculations, based on the approximation of the stationary state, to make them perform correctly. Basta discard the 3 responses that you encounter that are obviously erroneous if you apply the formula to determine the speed of a reaction.arrow_forwardFor the decomposition reaction of N2O5(g): 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 NO2 + NO3 (K1) | NO2 + NO3 → N2O5 (k-1) | NO2 + NO3 NO2 + O2 + NO (k2) | NO + N2O51 NO2 + NO2 + NO2 (K3) → Give the expression for the acceptable rate. → → (A). d[N205] dt == 2k,k₂[N₂O₂] k₁+k₁₂ (B). d[N2O5] =-k₁[N₂O] + k₁[NO₂] [NO3] - k₂[NO₂]³ dt (C). d[N2O5] =-k₁[N₂O] + k [NO] - k₂[NO] [NO] d[N2O5] (D). = dt = -k₁[N2O5] - k¸[NO][N₂05] dt Do not apply the calculations, based on the approximation of the stationary state, to make them perform correctly. Basta discard the 3 responses that you encounter that are obviously erroneous if you apply the formula to determine the speed of a reaction.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





