
(a)
Interpretation: Major products for the given set of reactions have to be found.
Concept Introduction:
The nitro group present in the compound is reduced to amino group by using Fe, Zn, Sn or SnCl2 in the presence of aqueous acid followed by the treatment with base such as NaOH. This reaction is more mild approach. It can be carried out in the presence of the other
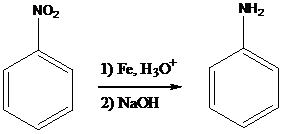
To find: Using a reduction followed by base treatment, prepare the major product of (a)
Find the reaction of the starting material of (a) with Fe, H3O+
(b)
Interpretation: Major products for the given set of reactions have to be found.
Concept Introduction:
Sodium cyanoborohydride is a strong reducing agent than sodium borohydride. It reduces the carbonyl group into amine group in a rapid way. So, it is called as reductive amination reactions.
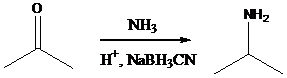
Aldehyde or ketone group is reacted with primary amine in the presence of sodium cyanoborohydride as a reducing agent and a proton source in the reaction medium to produce secondary amines.
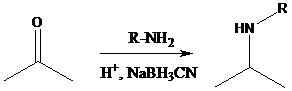
Aldehyde or ketone group is reacted with secondary amine in the presence of sodium cyanoborohydride as a reducing agent and a proton source in the reaction medium to produce tertiary amines.
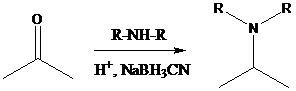
To find: Using a reductive amination, prepare the major product of (b)
Decide the reaction and the nature of the product
(c)
Interpretation: Major products for the given set of reactions have to be found.
Concept introduction:
The reduction of lithium aluminum hydride on nitriles followed by water work-up gives amine with an additional carbon atom in their chain. This type of reaction is applied to increase the carbon skeleton and change in the identity of the functional group.
To find: The major product of the reaction (c)
Give for the formation of lithium salt by reduction

(d)
Interpretation: Major products for the given set of reactions have to be found.
Concept introduction:
The reduction of lithium aluminum hydride on nitriles followed by water work-up gives amine with an additional carbon atom in their chain. This type of reaction is applied to increase the carbon skeleton and change in the identity of the functional group.
To find: The major product of the reaction (d)
Give for the formation of lithium ion by reduction
Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
- Macmillan Learning Alcohols can be oxidized by chromic acid derivatives. One such reagent is pyridinium chlorochromate, (C,H,NH*)(CICTO3), commonly known as PCC. Draw the proposed (neutral) intermediate and the organic product in the oxidation of 1-butanol by PCC when carried out in an anhydrous solvent such as CH₂C₁₂. PCC Intermediate OH CH2Cl2 Draw the intermediate. Select Draw Templates More с H Cr о Product Draw the product. Erase Select Draw Templates More H о Erasearrow_forwardIf I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. A C6H5 CH3arrow_forwardProvide the reagents for the following reactions.arrow_forward
- If I have 1-bromopropene, to obtain compound Z, I have to add two compounds A1 and A2. Indicate which compounds are needed. P(C6H5)3arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. O CH3CH2NH2, TSOH Select to Draw >arrow_forwardPredict the major organic product(s) for the following reaction.arrow_forward
- Predict the major organic product(s) for the following reactions.arrow_forwardProvide the complete mechanism for the reactions below. You must include appropriate arrows,intermediates, and formal charges.arrow_forwardIndicate the products obtained by reacting fluorobenzene with a sulfonitric mixture.arrow_forward
- If I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. C6H5 CH3arrow_forwardIf I have 1-bromopropene and I want to obtain (1,1-dipropoxyethyl)benzene, indicate the compound that I should add in addition to NaOH.arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Ο HSCH2CH2CH2SH, BF3 Select to Draw I Submitarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





