
(a)
Interpretation: Identification of the number of chirality centers in the given set of compounds
Concept Introduction:
There are two conditions to get a compound as a chiral. If an atom is attached with four different groups, that atom is having a chiral center. This is the first condition. The second one is that the compound doesn’t have any elements of symmetry. i.e., it should not have plane of symmetry and center of symmetry. If any one condition or both the conditions are possible in a compound, that compound is able to exhibit a chirality center. For example, the following compound has a chiral carbon.
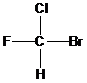
To find: Identify the number of chirality centers in the given compound (a)
Define a chirality center
(b)
Interpretation: Identification of the number of chirality centers in the given set of compounds
Concept Introduction:
There are two conditions to get a compound as a chiral. If an atom is attached with four different groups, that atom is having a chiral center. This is the first condition. The second one is that the compound doesn’t have any elements of symmetry. i.e., it should not have plane of symmetry and center of symmetry. If any one condition or both the conditions are possible in a compound, that compound is able to exhibit a chirality center. For example, the following compound has a chiral carbon.
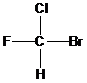
To find: Identify the number of chirality centers in the given compound (b)
Define a chirality center
(c)
Interpretation: Identification of the number of chirality centers in the given set of compounds
Concept Introduction:
There are two conditions to get a compound as a chiral. If an atom is attached with four different groups, that atom is having a chiral center. This is the first condition. The second one is that the compound doesn’t have any elements of symmetry. i.e., it should not have plane of symmetry and center of symmetry. If any one condition or both the conditions are possible in a compound, that compound is able to exhibit a chirality center. For example, the following compound has a chiral carbon.
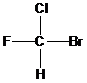
To find: Identify the number of chirality centers in the given compound (c)
Define a chirality center
Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
- Don't used hand raiting and don't used Ai solution and correct answerarrow_forwardH R Part: 1/2 :CI: is a/an electrophile Part 2 of 2 Draw the skeletal structure of the product(s) for the Lewis acid-base reaction. Include lone pairs and formal charges (if applicable) on the structures. 4-7: H ö- H Skip Part Check X :C1: $ % L Fi Click and drag to start drawing a structure. MacBook Pro & ㅁ x G 0: P Add or increase positive formal cha Save For Later Submit ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centearrow_forwardDraw the friedel-crafts acylation mechanism of m-Xylenearrow_forward
- Don't used hand raiting and don't used Ai solutionarrow_forward1. Base on this experimental results, how do you know that the product which you are turning in is methyl 3-nitrobenzoate(meta substituted product ) rather than either of the other two products? 2. What observation suggests that at least a small amount of one or both of the other two isomers are in the mother liquor?arrow_forwardExplain Huckel's rule.arrow_forward
- here is my question can u help me please!arrow_forwardSo I need help with understanding how to solve these types of problems. I'm very confused on how to do them and what it is exactly, bonds and so forth that I'm drawing. Can you please help me with this and thank you very much!arrow_forwardSo I need help with this problem, can you help me please and thank you!arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





