
Concept explainers
(a)
Interpretation:
Distribution of d-electrons and the geometry of the complex ion
Concept Introduction:
Complex compounds exist in following geometries - tetrahedral, square planar, octahedral etc.
When ligands approach the metal ion the degeneracy in d-orbitals of the metal ion is destroyed and they split into two different energy levels. In case of tetrahedral complex, the
In case of square planar complex, the 5 d-orbitals split into following pattern and it is given below with increasing order of energy.
(a)
Answer to Problem 22.60QP
The d-electrons are distributed in the complex ion
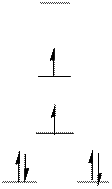
The complex has square planar geometry.
Explanation of Solution
In the complex ion

The distribution of d-electrons as shown above correlates to that of square planar geometry. Hence the complex ion
Splitting of d-orbitals determines the geometry of the complex.
(b)
Interpretation:
Distribution of d-electrons and the geometry of the complex ion
Concept Introduction:
Complex compounds exist in following geometries - tetrahedral, square planar, octahedral etc.
When ligands approach the metal ion the degeneracy in d-orbitals of the metal ion is destroyed and they split into two different energy levels. In case of tetrahedral complex, the
In case of square planar complex, the 5 d-orbitals split into following pattern and it is given below with increasing order of energy.
(b)
Answer to Problem 22.60QP
The d-electrons are distributed in the complex ion
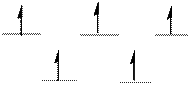
The complex has tetrahedral geometry.
Explanation of Solution
In the complex ion
Atomic number of Manganese is

The distribution of d-electrons as shown above correlates to that of tetrahedral geometry. Hence the complex ion
Splitting of d-orbitals determines the geometry of the complex.
(c)
Interpretation:
Distribution of d-electrons and the geometry of the complex ion
Concept Introduction:
Complex compounds exist in following geometries - tetrahedral, square planar, octahedral etc.
When ligands approach the metal ion the degeneracy in d-orbitals of the metal ion is destroyed and they split into two different energy levels. In case of tetrahedral complex, the
In case of square planar complex, the 5 d-orbitals split into following pattern and it is given below with increasing order of energy.
(c)
Answer to Problem 22.60QP
The d-electrons are distributed in the complex ion
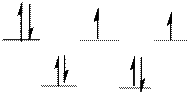
The complex has tetrahedral geometry.
Explanation of Solution
In the complex ion
Atomic number of Nickel is
The distribution of d-electrons as shown above correlates to that of tetrahedral geometry. Hence the complex ion
Splitting of d-orbitals determines the geometry of the complex.
(d)
Interpretation:
Distribution of d-electrons and the geometry of the complex ion
Concept Introduction:
Complex compounds exist in following geometries - tetrahedral, square planar, octahedral etc.
When ligands approach the metal ion the degeneracy in d-orbitals of the metal ion is destroyed and they split into two different energy levels. In case of tetrahedral complex, the
In case of square planar complex, the 5 d-orbitals split into following pattern and it is given below with increasing order of energy.
(d)
Answer to Problem 22.60QP
The d-electrons are distributed in the complex ion

The complex has square planar geometry.
Explanation of Solution
In the complex ion
Atomic number of Gold is

The distribution of d-electrons as shown above correlates to that of square planar geometry. Hence the complex ion
Splitting of d-orbitals determines the geometry of the complex.
Want to see more full solutions like this?
Chapter 22 Solutions
Bundle: General Chemistry, Loose-Leaf Version, 11th + LabSkills PreLabs v2 for Organic Chemistry (powered by OWLv2), 4 terms (24 months) Printed ... for Ebbing/Gammon's General Chemistry, 11th
- Write the molecular formula for a compound with the possible elements C, H, N and O that exhibits a molecular ion at M+ = 85.0899. Exact Masses of the Most Abundant Isotope of Selected Elements Isotope Natural abundance (%) Exact mass 1H 99.985 1.008 12C 98.90 12.000 14N 99.63 14.003 160 99.76 15.995 Molecular formula (In the order CHNO, with no subscripts)arrow_forwardUse the data below from an electron impact mass spectrum of a pure compound to deduce its structure. Draw your structure in the drawing window. Data selected from the NIST WebBook, https://webbook.nist.gov/chemistry/ m/z Relative intensity 59 3.0 58 64 43 100 15 23 • You do not have to consider stereochemistry. •You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. + n[] 85 // ? CH4 Previous Nextarrow_forwardWrite the molecular formula for a compound with the possible elements C, H, N and O that exhibits a molecular ion at M* = 128.0632. Exact Masses of the Most Abundant Isotope of Selected Elements Isotope Natural abundance (%) Exact mass 1H 99.985 12C 98.90 14N 99.63 160 99.76 Molecular formula 1.008 12.000 14.003 15.995 (In the order CHNO, with no subscripts)arrow_forward
- Can I please get help with this? And can I please the lowest possible significant number?arrow_forwardWhat is the molar mass of a gas that takes three times longer to effuse than helium?arrow_forwardFirst image: I have to show the mecanism (with arows and structures) of the reaction at the bottom. Also I have to show by mecanism why the reaction wouldn't work if the alcohol was primary Second image: I have to show the mecanism (with arrows and structures) for the reaction on the left, where the alcohol A is added fast in one portion its not an examarrow_forward
- what is the skeletal structure of a tertiary alkyl fluoride with six carbon atoms and no rings.arrow_forwardOne step of glycolysis is a retro-aldol reaction (aldolase) to produce ATP.Below is the aldol reaction of the equilibrium. Show the mechanism for the base catalyzed reaction. *see imagearrow_forwardProvide the missing information. *see imagearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





