
Concept explainers
(a)
Interpretation:
Whether the given compound is synthesized in good yield or not by an aldol condensation with its starting materials is to be stated. The reason as to why the required aldol condensation would not succeed for compound that is not synthesized in good yield is to be stated.
Concept Introduction:
Aldol condensation is the nucleophilic addition reaction of the carbonyl compounds. An enolate ion generated from one of the carbonyl compound attack the electrophilic carbon of the other carbonyl compound to produce
Answer to Problem 22.22P
The given compound can be synthesized using aldol condensation in good yield. The starting materials are
Explanation of Solution
The given compound is shown below.
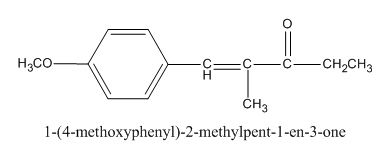
Figure 1
In the above preparation of the compound, the enolate is generated on the
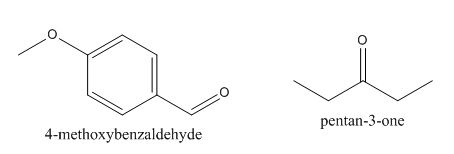
Figure 2
The given compound can be synthesized by aldol condensation of
(b)
Interpretation:
Whether the given compound is synthesized in good yield or not by an aldol condensation with its starting materials is to be stated. The reason as to why the required aldol condensation would not succeed for compound that is not synthesized in good yield is to be stated.
Concept Introduction:
Aldol condensation is the nucleophilic addition reaction of the carbonyl compounds. An enolate ion generated from one of the carbonyl compound attack the electrophilic carbon of the other carbonyl compound to produce
Answer to Problem 22.22P
The given compound can be synthesized using aldol condensation in good yield. The starting materials are benzaldehyde and
Explanation of Solution
The given compound is shown below.
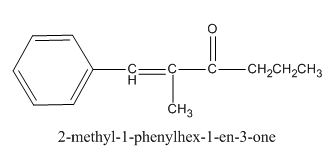
Figure 3
In the preparation of the above compound, the enolate will be generated on the
The structures of the starting materials which are used to produce the given compound are shown below.
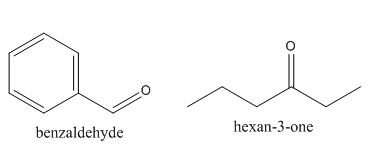
Figure 4
The given compound can be synthesized by aldol condensation of benzaldehyde and
(c)
Interpretation:
Whether the given compound is synthesized in good yield or not by an aldol condensation with its starting materials is to be stated. The reason as to why the required aldol condensation would not succeed for compound that is not synthesized in good yield is to be stated.
Concept Introduction:
Aldol condensation is the nucleophilic addition reaction of the carbonyl compounds. An enolate ion generated from one of the carbonyl compound attack the electrophilic carbon of the other carbonyl compound to produce
Answer to Problem 22.22P
The given compound can be synthesized using aldol condensation but not in a good in good yield. The starting material is
Explanation of Solution
The given compound is shown below.
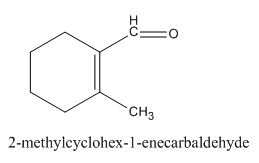
Figure 5
In the preparation of the above compound, intramolecular aldol condensation takes place. To form this compound enolate must be generated by aldehyde and it has to attack the carbonyl carbon of ketone which is not favorable condition. Favorable condition is that enolate should be generated on ketone which attacks the electrophilic carbon of the aldehyde. This is because the carbon of aldehyde is more acidic than the carbon of ketone. The structure of the starting material which is used to produce the given compound is shown below.
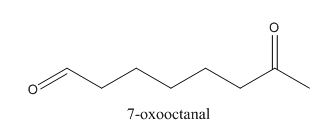
Figure 6
Therefore, the given compound cannot be synthesized by aldol condensation in a good yield.
This compound cannot be synthesized in a good yield by the aldol condensation.
(d)
Interpretation:
Whether the given compound is synthesized in good yield or not by an aldol condensation with its starting materials is to be stated. The reason as to why the required aldol condensation would not succeed for compound that is not synthesized in good yield is to be stated.
Concept Introduction:
Aldol condensation is the nucleophilic addition reaction of the carbonyl compounds. An enolate ion generated from one of the carbonyl compound attack the electrophilic carbon of the other carbonyl compound to produce
Answer to Problem 22.22P
The given compound can be synthesized by aldol condensation in good yield. The starting material is
Explanation of Solution
The given compound is shown below.
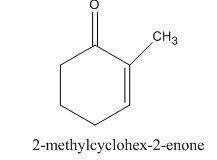
Figure 7
The given compound, is prepared by the intramolecular aldol condensation of
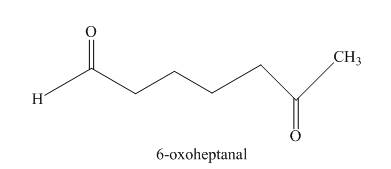
Figure 8
The given compound can be synthesized by intramolecular aldol condensation of
(e)
Interpretation:
Whether the given compound is synthesized in good yield or not by an aldol condensation with its starting materials is to be stated. The reason as to why the required aldol condensation would not succeed for compound that is not synthesized in good yield is to be stated.
Concept Introduction:
Aldol condensation is the nucleophilic addition reaction of the carbonyl compounds. An enolate ion generated from one of the carbonyl compound attack the electrophilic carbon of the other carbonyl compound to produce
Answer to Problem 22.22P
The given compound can be synthesized from aldol condensation in a good yield. The starting materials are:
Explanation of Solution
The given compound is shown below.
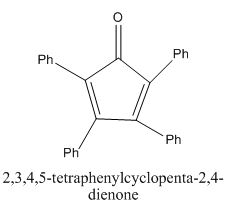
Figure 9
In the preparation of the given compound, the enolate will be generated on the
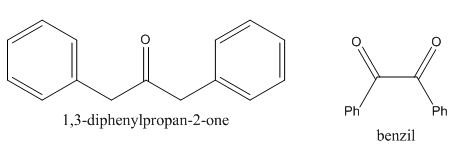
Figure 10
The given compound can be synthesized by aldol condensation of
(f)
Interpretation:
Whether the given compound is synthesized in good yield or not by an aldol condensation with its starting materials is to be stated. The reason as to why the required aldol condensation would not succeed for compound that is not synthesized in good yield is to be stated.
Concept Introduction:
Aldol condensation is the nucleophilic addition reaction of the carbonyl compounds. An enolate ion generated from one of the carbonyl compound attack the electrophilic carbon of the other carbonyl compound to produce
Answer to Problem 22.22P
The given compound can be synthesized by aldol condensation in a good yield. The starting materials are
Explanation of Solution
The given compound is shown below.
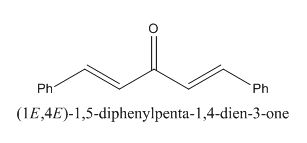
Figure 11
In the preparation of the compound, the enolate is generated on the
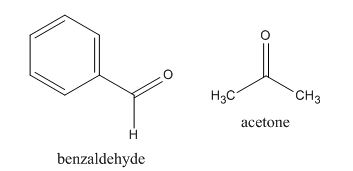
Figure 12
The given compound can be synthesized by aldol condensation of benzaldehyde and acetone.
(g)
Interpretation:
Whether the given compound is synthesized in good yield or not by an aldol condensation with its starting materials is to be stated. The reason as to why the required aldol condensation would not succeed for compound that is not synthesized in good yield is to be stated.
Concept Introduction:
Aldol condensation is the nucleophilic addition reaction of the carbonyl compounds. An enolate ion generated from one of the carbonyl compound attack the electrophilic carbon of the other carbonyl compound to produce
Answer to Problem 22.22P
The given compound can be synthesized by aldol condensation. The starting materials are trimethylacetone and acetaldehyde.
Explanation of Solution
The given compound is shown below.
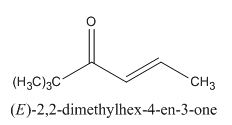
Figure 13
In the preparation of the given compound, the enolate will be generated on the
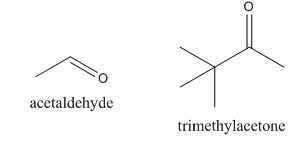
Figure 14
The given compound can be synthesized by aldol condensation by trimethylacetone and acetaldehyde in a good yield.
(h)
Interpretation:
Whether the given compound is synthesized in good yield or not by an aldol condensation with its starting materials is to be stated. The reason as to why the required aldol condensation would not succeed for compound that is not synthesized in good yield is to be stated.
Concept Introduction:
Aldol condensation is the nucleophilic addition reaction of the carbonyl compounds. An enolate ion generated from one of the carbonyl compound attack the electrophilic carbon of the other carbonyl compound to produce
Answer to Problem 22.22P
The given compound can be synthesized by aldol condensation in a good yield. The starting material is
Explanation of Solution
The given compound is shown below.
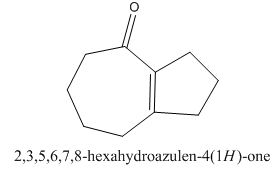
Figure 15
The enolate will be generated on the
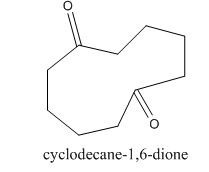
Figure 16
The given compound can be synthesized by aldol condensation of
Want to see more full solutions like this?
Chapter 22 Solutions
Organic Chemistry Study Guide and Solutions
- Synthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- If possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forward
- We mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forward
- Indicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forwardIndicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are mixed with sodium ethoxide in ethanol.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
