
Concept explainers
(a)
Interpretation:
The class of enzyme catalyzing the given reaction needs to be determined.
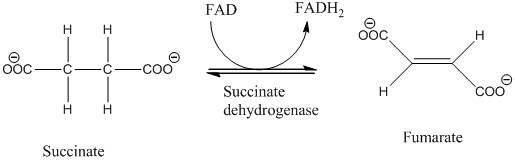
Concept Introduction:
Enzymes are protein molecules of high molecular mass and forms colloidal solution in water, and enzymes is produce to increases the rate of virtually all
(b)
Interpretation:
The class of enzyme catalyzed of the given reaction needs to be determined.
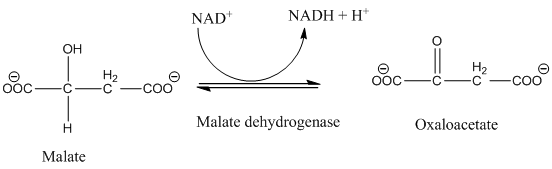
Concept Introduction:
Enzymes are protein molecules of high molecular mass and forms colloidal solution in water, and enzymes is produce to increases the rate of virtually all chemical reactions within cells by the active site of proteins. Enzymes increase the rate of reaction at about
Want to see the full answer?
Check out a sample textbook solution
Chapter 21 Solutions
General, Organic, & Biological Chemistry
- 7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward7 Comment on the general features of the predicted (extremely simplified) ¹H- NMR spectrum of lycopene that is provided below. 00 6 57 PPM 3 2 1 0arrow_forwardIndicate the compound formula: dimethyl iodide (propyl) sulfonium.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning




