
General, Organic, & Biological Chemistry
3rd Edition
ISBN: 9780073511245
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 21, Problem 21.41P
(a) Identify the amino acid shown with all uncharged atoms in the ball-and-stick model. (b) Give the three-letter and one-letter abbreviations for the amino acid. (c) Draw the form present at the isoelectric point of the amino acid. 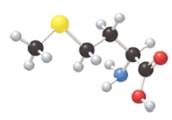
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
(a) Identify the amino acid shown with all uncharged atoms in the ball-and-stick model. (b) Give the three-letter and one-letter abbreviations for the amino acid. (c) Draw the form present at the isoelectric point of the amino acid.
(a) Draw the structure of the two possible dipeptides that can be formed by combining valine and phenylalanine. (b) In each dipeptide, label the N- and C-terminal amino acids. (c) Name each peptide using three-letter symbols.
With reference to the following peptide: (a) Identify the N-terminal and Cterminal amino acids. (b) Name the peptide using one-letter abbreviations. (c) Label all the amide bonds in the peptide backbone.
Chapter 21 Solutions
General, Organic, & Biological Chemistry
Ch. 21.2 - In addition to the amino and carboxyl groups, what...Ch. 21.2 - Draw both enantiomers of each amino acid in...Ch. 21.2 - Prob. 21.3PCh. 21.3 - Draw the structure of the amino acid valine at...Ch. 21.3 - Identify the amino acid shown with all uncharged...Ch. 21.4 - Identify the N-terminal and C-terminal amino acid...Ch. 21.4 - Prob. 21.7PCh. 21.4 - Prob. 21.8PCh. 21.4 - Prob. 21.9PCh. 21.4 - Prob. 21.10P
Ch. 21.4 - Prob. 21.11PCh. 21.5 - Prob. 21.12PCh. 21.6 - Prob. 21.13PCh. 21.6 - Prob. 21.14PCh. 21.6 - Prob. 21.15PCh. 21.7 - Why is hemoglobin more water soluble than ...Ch. 21.8 - Prob. 21.17PCh. 21.8 - Prob. 21.18PCh. 21.9 - Prob. 21.19PCh. 21.9 - Prob. 21.20PCh. 21.9 - Prob. 21.21PCh. 21.9 - Prob. 21.22PCh. 21.10 - Prob. 21.23PCh. 21.10 - Prob. 21.24PCh. 21.10 - Prob. 21.25PCh. 21.10 - Prob. 21.26PCh. 21.10 - Prob. 21.27PCh. 21.10 - Prob. 21.28PCh. 21.10 - Prob. 21.29PCh. 21.11 - Prob. 21.30PCh. 21 - The amino acid alanine is a solid at room...Ch. 21 - Why is phenylalanine water soluble but...Ch. 21 - Draw the structure of a naturally occurring amino...Ch. 21 - Draw the structure of a naturally occurring amino...Ch. 21 - For each amino acid: [1] draw the L enantiomer in...Ch. 21 - For each amino acid: [1] draw the L enantiomer in...Ch. 21 - Draw both enantiomers of each amino acid and label...Ch. 21 - Which of the following Fischer projections...Ch. 21 - For each amino acid: [1] give the name; [2] give...Ch. 21 - For each amino acid: [1] give the name; [2] give...Ch. 21 - (a) Identify the amino acid shown with all...Ch. 21 - Prob. 21.42PCh. 21 - Prob. 21.43PCh. 21 - Draw the structure of the neutral, positively...Ch. 21 - Prob. 21.45PCh. 21 - Prob. 21.46PCh. 21 - (a) Draw the structure of the two possible...Ch. 21 - Prob. 21.48PCh. 21 - Prob. 21.49PCh. 21 - For each tripeptide: [1] draw the structure of the...Ch. 21 - Prob. 21.51PCh. 21 - For each tripeptide: [1] identify the amino acids...Ch. 21 - Prob. 21.53PCh. 21 - Prob. 21.54PCh. 21 - Prob. 21.55PCh. 21 - Prob. 21.56PCh. 21 - Prob. 21.57PCh. 21 - Prob. 21.58PCh. 21 - Prob. 21.59PCh. 21 - Prob. 21.60PCh. 21 - Prob. 21.61PCh. 21 - Prob. 21.62PCh. 21 - What type of intermolecular forces exist between...Ch. 21 - What type of interaction occur at each of the...Ch. 21 - Prob. 21.65PCh. 21 - Draw the structures of the amino acids tyrosine...Ch. 21 - Prob. 21.67PCh. 21 - Prob. 21.68PCh. 21 - Prob. 21.69PCh. 21 - Prob. 21.70PCh. 21 - Prob. 21.71PCh. 21 - Hydrogen bonding stabilizes both the secondary and...Ch. 21 - Prob. 21.73PCh. 21 - Prob. 21.74PCh. 21 - Prob. 21.75PCh. 21 - Prob. 21.76PCh. 21 - What class of enzyme catalyzes each of the...Ch. 21 - What class of enzyme catalyzes each of the...Ch. 21 - Prob. 21.79PCh. 21 - Prob. 21.80PCh. 21 - Prob. 21.81PCh. 21 - What kind of reaction is catalyzed by each of the...Ch. 21 - Prob. 21.83PCh. 21 - How will each of the following changes affect the...Ch. 21 - Prob. 21.85PCh. 21 - Prob. 21.86PCh. 21 - Prob. 21.87PCh. 21 - Prob. 21.88PCh. 21 - Prob. 21.89PCh. 21 - Prob. 21.90PCh. 21 - Prob. 21.91PCh. 21 - Prob. 21.92PCh. 21 - Why must vegetarian diets be carefully balanced?Ch. 21 - Prob. 21.94PCh. 21 - Sometimes an incision is cauterized (burned) to...Ch. 21 - Why is insulin administered by injection instead...Ch. 21 - Prob. 21.97PCh. 21 - The silk produced by a silkworm is a protein with...Ch. 21 - Explain the difference in the mechanism of action...Ch. 21 - Prob. 21.100PCh. 21 - Prob. 21.101CPCh. 21 - Suggest a reason for the following observation....
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Identify the R group of the side chain in the following amino acids that results in the side-chain classification indicated in parentheses see Table 19.1: a. tyrosine neutral, polar b. glutamate acidic, polar c. methionine neutral, nonpolar d. histidine basic, polar e. cysteine neutral, polar f. valine neutral, nonpolararrow_forwardAt room temperature, amino acids are solids with relatively high decomposition points. Explain why.arrow_forwardWhich is NOT a characteristic of proteins? a. They contain genetic information. b. They can act as hormones. c. They can catalyze chemical reactions. d. They act in cell membrane trafficking.arrow_forward
- 5. For each of the following modified amino acid side chains, iden- tify the amino acid from which it was derived and the type of chemical modification that has occurred. (a) –CH,OPO, (b) –CH,CH1CO0©2, (c) – 1CH2,-NH-C102CH3arrow_forwardcan methylguanidoacetic acid (creatine) be classified as a carbohydrate, lipd or residue of a protein - explain why it can or can not.arrow_forward10. Which of the following protein structures does denaturation destroy (a) Primary structure only (b) Secondary structure only (c) Tertiary structure only (d) Quaternary structure only (e) Secondary, tertiary, and quaternary structuresarrow_forward
- Write only the three letter abbreviation of the amino acid pictured. SH CH, O H,N--C-C-OH H.arrow_forwardName each peptide using both the three-letter and one-letterabbreviations of the component amino acids.arrow_forwardIdentify the amino acid shown with all uncharged atoms in the ball-and-stick model, and draw the neutral, positively charged, and negatively charged forms of the amino acid.arrow_forward
- In relation to the spatial structure of the proteins select the INCORRECT alternative: (a) The quaternary structure of proteins consists of the association of two or more protein chains to form the complete protein. (b) The tertiary structure of proteins is mainly due to long-range interactions between amino acids that are far apart in the protein sequence but spatially close due to protein coiling. (c) Disulfide bridges are produced or broken as a result of oxidation-reduction processes. (d) The interactions between peptide chains in the secondary structure of proteins are basically due to Van der Waals forces. (e) The primary structure of proteins is basically due to the existence of covalent bonds between the different amino acids that constitute them.arrow_forwardWhich of the following amino acids are typically found in the interior of a globular protein, and which are typically found on the surface: (a) phenylalanine; (b) aspartic acid; (c) lysine; (d) isoleucine; (e) arginine; (f) glutamic acid?arrow_forwardProvide the correct three - letter abbreviation for the following amino. acid: Provide the correct three-letter abbreviation for the following amino acid: O || H₂N-CH-C-OH I CH3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Biomolecules - Protein - Amino acids; Author: Tutorials Point (India) Ltd.;https://www.youtube.com/watch?v=ySNVPDHJ0ek;License: Standard YouTube License, CC-BY