
Concept explainers
For each amino acid: [1] draw the L enantiomer in a Fischer projection; [2] classify the amino acid as neutral, acidic, or basic; [3] give the three-letter symbol; [4] give the one-letter symbol.
- leucine
- tryptophan
- lysine
- aspartic acid
(a)
Interpretation:
The L-isomer of leucine in Fisher projection needs to be drawn and should be identified as acidic, basic or neutral. The three letter and one symbol for it needs to be determined.
Concept Introduction:
Like monosaccharides, the prefixes D and L are used to designate the arrangement of groups to the chiral center of amino acid, when drawn with a vertical carbon chain having
Amino acids that have the
Answer to Problem 21.35P
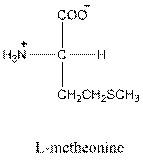
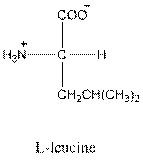
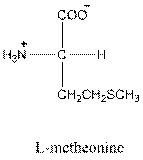
- L-isomer of leucine is as follows:
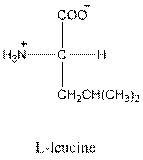
- Leucine is neutral.
- Three-letter symbol for leucine is Leu.
- One-letter symbol for leucine is L.
Explanation of Solution
For leucine the R group is
The amino acid leucine has one positive charge on amino group
One letter symbol for leucine is L and three-letter symbol for leucine is Leu.
(b)
Interpretation:
To draw L-isomer of tryptophan (amino acid) in Fisher projection, to identify it is acidic, basic or neutral and to give three letter and one symbol for it
Concept Introduction : :
Like monosaccharides, the prefixes D and L are used to designate the arrangement of groups to the chiral center of amino acid, when drawn with a vertical carbon chain having
Amino acids that have the
Answer to Problem 21.35P
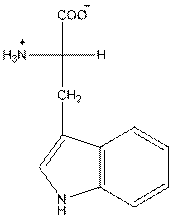
- L-isomer of tryptophan is as follows:
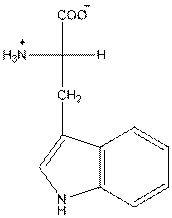
- Amino acid tryptophan is neutral.
Explanation of Solution
For tryptophan the R group is as follows:
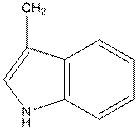
And in L-isomer the amino group
The amino acid tryptophan has one positive charge on amino group
One letter symbol for tryptophan is W and three-letter symbol for tryptophan is Trp.
(c)
Interpretation:
The L-isomer of lysine in Fisher projection needs to be drawn, whether it is acidic, basic or neutral needs to be determined and the three-letter and one symbol for it needs to be given.
Concept Introduction:
Like monosaccharides, the prefixes D and L are used to designate the arrangement of groups to the chiral center of amino acid, when drawn with a vertical carbon chain having
Amino acids that have the
Answer to Problem 21.35P
- L-isomer of lysine is as follows:
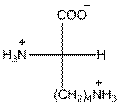
- The amino acid lysine is basic in nature.
- The three letter symbol for lysine is Lys.
- The one letter symbol for lysine is K.
Explanation of Solution
For lysine the R group is
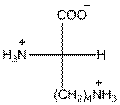
The amino acid lysine has one positive charge on amino group
The three letter symbol for lysine is Lys and one letter symbol for lysine is K.
(d)
Interpretation:
The L-isomer of aspartic acid in Fisher projection needs to be drawn, whether it is acidic, basic or neutral needs to be determined and the three-letter and one symbol for it needs to be given.
Concept Introduction:
Like monosaccharides, the prefixes D and L are used to designate the arrangement of groups to the chiral center of amino acid, when drawn with a vertical carbon chain having
Amino acids that have the
Answer to Problem 21.35P
- L-isomer of aspartic acid is as follows:
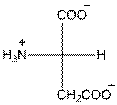
- The aspartic acid is acidic in nature.
- The three letter symbol for aspartic acid is Asp.
- The one letter symbol for aspartic acid is D.
Explanation of Solution
For aspartic acid the R group is
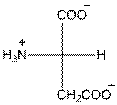
The aspartic acid has one positive charge on amino group
The three letter symbol for aspartic acid is Asp and the one letter symbol for aspartic acid is D.
Want to see more full solutions like this?
Chapter 21 Solutions
General, Organic, & Biological Chemistry
- Draw the stepwise mechanism for the reactionsarrow_forwardPart I. a) Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone b) Pinacol (2,3-dimethyl, 1-3-butanediol) on treatment w/ acid gives a mixture of pina colone (3,3-dimethyl-2-butanone) and 2, 3-dimethyl - 1,3-butadiene. Give reasonable mechanism the formation of the products Forarrow_forward3. The explosive decomposition of 2 mole of TNT (2,4,6-trinitrotoluene) is shown below: Assume the C(s) is soot-basically atomic carbon (although it isn't actually atomic carbon in real life). 2 CH3 H NO2 NO2 3N2 (g)+7CO (g) + 5H₂O (g) + 7C (s) H a. Use bond dissociation energies to calculate how much AU is for this reaction in kJ/mol.arrow_forward
- Part I. Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone and answer the ff: Pinacol (2,3-dimethyl, 1-3-butanediol) on treatment w/ acid gives a mixture of pina colone and (3,3-dimethyl-2-butanone) 2,3-dimethyl-1,3-butadiene. Give reasonable mechanism the formation of the products Forarrow_forwardShow the mechanism for these reactionsarrow_forwardDraw the stepwise mechanismarrow_forward
- Draw a structural formula of the principal product formed when benzonitrile is treated with each reagent. (a) H₂O (one equivalent), H₂SO₄, heat (b) H₂O (excess), H₂SO₄, heat (c) NaOH, H₂O, heat (d) LiAlH4, then H₂Oarrow_forwardDraw the stepwise mechanism for the reactionsarrow_forwardDraw stepwise mechanismarrow_forward
- Part I. Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone and answer the ff: a) Give the major reason for the exposure of benzophenone al isopropyl alcohol (w/acid) to direct sunlight of pina colone Mechanism For b) Pinacol (2,3-dimethy 1, 1-3-butanediol) on treatment w/ acid gives a mixture (3,3-dimethyl-2-butanone) and 2, 3-dimethyl-1,3-butadiene. Give reasonable the formation of the productsarrow_forwardwhat are the Iupac names for each structurearrow_forwardWhat are the IUPAC Names of all the compounds in the picture?arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





