
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2.12, Problem 19P
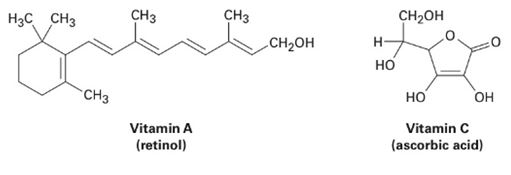
Of the two vitamins A and C, one is hydrophilic and water-soluble while the other is hydrophobic and fat-soluble. Which is which?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Rank the following compounds in order of decreasing boiling point.
ннннн
-С-С-Н
.
н-с-
ННННН
H
ΗΤΗ
НННН
TTTĪ
н-с-с-с-с-о-н
НННН
НН
C' Н
н-с-с-с-с-н
НН
||
Ш
НННН
H-C-C-C-C-N-H
ННННН
IV
Rank the following compounds in order of decreasing dipole moment.
|>||>|||
||>|||>|
|>|||>||
|||>||>|
O ||>>|||
H
F
H
F
H
c=c
||
H
c=c
F F
III
choose the description that best describes the geometry for the following charged species ch3-
Chapter 2 Solutions
Organic Chemistry
Ch. 2.1 - Prob. 1PCh. 2.1 - Prob. 2PCh. 2.1 - Use the electronegativity values shown in Figure...Ch. 2.1 - Look at the following electrostatic potential map...Ch. 2.2 - Ethylene glycol, HOCH2CH2OH, may look nonpolar...Ch. 2.2 - Make three-dimensional drawings of the following...Ch. 2.3 - Calculate formal charges for the nonhydrogen atoms...Ch. 2.3 - Organic phosphate groups occur commonly in...Ch. 2.6 - Which of the following pairs of structures...Ch. 2.6 - Draw the indicated number of resonance forms for...
Ch. 2.7 - Nitric acid (HNO3) reacts with ammonia (NH3) to...Ch. 2.8 - Prob. 12PCh. 2.8 - Amide ion, H2N-, is a much stronger base than...Ch. 2.9 - Prob. 14PCh. 2.9 - Prob. 15PCh. 2.9 - Prob. 16PCh. 2.11 - Using curved arrows, show how the species in part...Ch. 2.11 - Prob. 18PCh. 2.12 - Of the two vitamins A and C, one is hydrophilic...Ch. 2.SE - Prob. 20VCCh. 2.SE - The following model is a representation of...Ch. 2.SE - cis-l, 2-Dichloroethylene and trans-1,...Ch. 2.SE - The following molecular models are representations...Ch. 2.SE - Predict the product(s) of the acid/base reactions...Ch. 2.SE - Use curved arrows to draw the protonated form of...Ch. 2.SE - Prob. 26MPCh. 2.SE - Double bonds can also act like Lewis bases,...Ch. 2.SE - Prob. 28APCh. 2.SE - Use the electronegativity table given in Figure...Ch. 2.SE - Which of the following molecules has a dipole...Ch. 2.SE - Prob. 31APCh. 2.SE - Phosgene, C12C=O, has a smaller dipole moment than...Ch. 2.SE - Prob. 33APCh. 2.SE - Methanethiol, CH3SH, has a substantial dipole...Ch. 2.SE - Calculate the formal charges on the atoms shown in...Ch. 2.SE - Assign formal charges to the atoms in each of the...Ch. 2.SE - Which of the following pairs of structures...Ch. 2.SE - Prob. 38APCh. 2.SE - 1, 3-Cyclobutadiene is a rectangular molecule with...Ch. 2.SE - Alcohols can act either as weak acids or as weak...Ch. 2.SE - The O-H hydrogen in acetic acid is more acidic...Ch. 2.SE - Draw electron-dot structures for the following...Ch. 2.SE - Write the products of the following acid-base...Ch. 2.SE - Rank the following substances in order of...Ch. 2.SE - Which, if any, of the substances in Problem 2-44...Ch. 2.SE - The ammonium ion (NH4+, pKa = 9.25) has a lower...Ch. 2.SE - Prob. 47APCh. 2.SE - Prob. 48APCh. 2.SE - Calculate Ka values from the following pka’s:...Ch. 2.SE - Calculate pKa values from the following Ka’s:...Ch. 2.SE - What is the pH of a 0.050 M solution of formic...Ch. 2.SE - Prob. 52APCh. 2.SE - Maleic acid has a dipole moment, but the closely...Ch. 2.SE - Assume that you have two unlabeled bottles, one of...Ch. 2.SE - Identify the acids and bases in the following...Ch. 2.SE - Which of the following pairs represent resonance...Ch. 2.SE - Draw as many resonance structures as you can for...Ch. 2.SE - Carbocations, which contain a trivalent,...Ch. 2.SE - We’ll see in the next chapter that organic...Ch. 2.SE - The azide functional group, which occurs in...Ch. 2.SE - Phenol, C6H5OH, is a stronger acid than methanol,...Ch. 2.SE - Thiamin diphosphate (TPP), a derivative of vitamin...Ch. 2.SE - Determine if each compound or ion below has a...Ch. 2.SE - Prob. 64APCh. 2.SE - Prob. 65APCh. 2.SE - Draw the conjugate base for each compound below...Ch. 2.SE - 1, 1, 1-Trichloroethanol is an acid more than 1000...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Why isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forwardWhat is the approximate bond angle around the nitrogen atom? HNH H Harrow_forwardOH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forward
- Complete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all species within the reaction. S₂O₃²⁻(aq) → S₄O₆²⁻(aq)arrow_forwardQ Select to Edit NH3 (CH3)2CHCI (1 equiv) AICI 3 Select to Draw cat. H2SO4 SO3 (1 equiv) HO SOCl2 pyridine Select to Edit >arrow_forwardComplete and balance the following half-reaction in basic solution. Be sure to include the proper phases for all species within the reaction. Zn(s) → Zn(OH)₄²⁻(aq)arrow_forward
- b. ὋΗ CH3CH2OH H2SO4arrow_forwardFor the reaction A (g) → 3 B (g), Kp = 0.379 at 298 K. What is the value of ∆G for this reaction at 298 K when the partial pressures of A and B are 5.70 atm and 0.250 atm?arrow_forward14. Calculate the concentrations of Ag+, Ag(S2O3), and Ag(S2O3)23- in a solution prepared by mixing 150.0 mL of 1.00×10-3 M AgNO3 with 200.0 mL of 5.00 M Na2S2O3 Ag+ + S20 Ag(S203)¯ K₁ = 7.4 × 108 Ag(S203)¯ + S20¯ = Ag(S203) K₂ = 3.9 x 104arrow_forward
- ΗΝ, cyclohexanone pH 4-5 Draw Enamine I I CH3CH2Br THF, reflux H3O+ I Drawing Draw Iminium Ionarrow_forward:0: :0: Select to Add Arrows :0: (CH3)2NH :0: ■ Select to Add Arrows :0: :0: (CH3)2NH ■ Select to Add Arrowsarrow_forwardDraw the product of the following H action sequence. Ignore any inorganic byproducts formed. 1. (CH3CH2)2CuLi, THF 2. CH3Br Q Atoms, Bonds and Rings H Charges ㅁarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY