
Concept explainers
a)
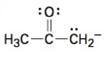
Interpretation:
To draw the maximum resonance structures possible for the species

Concept introduction:
Resonance forms differ only in the placement of their π and nonbonding valence electrons. Neither the position nor the hybridization of any atom changes from one resonance form to another. For writing different resonance forms in the structure given, first a three atom groupings with a multiple bond and a p orbital with pair of electrons is to be identified. Then the exchange of position of double bond and electrons in p orbital will give another resonance form. The shift is represented by a curved arrow.
To draw:
The maximum resonance structures possible for the species

Answer to Problem 38AP
The maximum resonance structures possible for the species  is two.
is two.
Explanation of Solution
Resonance forms differ only in the placement of their π and their nonbonding valence electrons. Neither the position nor the hybridization of any atom changes from one resonance form to another. The anion has a carbon atom doubly bonded to an oxygen and singly bonded to an adjacent carbon atom bearing negative charge. Using the nonbonding electrons on the negatively charged carbon atom and the π bond one more structure can be drawn, as shown, without change in position or hybridization of any atom. Hence the species given has two resonance forms.
The maximum resonance structures possible for the species  are two.
are two.
b)
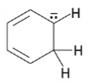
Interpretation:
To draw the maximum resonance structures possible for the species

Concept introduction:
Resonance forms differ only in the placement of their π and nonbonding valence electrons. Neither the position nor the hybridization of any atom changes from one resonance form to another. For writing different resonance forms in the structure given, first a three atom groupings with a multiple bond and a p orbital with pair of electrons is to be identified. Then the exchange of position of double bond and electrons in p orbital will give another resonance form. The shift is represented by a curved arrow.
To draw:
The maximum resonance structures possible for the species

Answer to Problem 38AP
The maximum resonance structures possible for the species  is three.
is three.
Explanation of Solution
Resonance forms differ only in the placement of their π and their nonbonding valence electrons. Neither the position nor the hybridization of any atom changes from one resonance form to another. The anion given has a carbon atom doubly bonded to another carbon and singly bonded to an adjacent carbon atom bearing negative charge. Another double bond also is present. Using the nonbonding electrons on the negatively charged carbon atom and the π bonds two more structures can be drawn as shown, without change in position or hybridization of any atom. Hence the species given has three resonance forms.
Conclusion:
The maximum resonance structures possible for the species  are three.
are three.
The maximum resonance structures possible for the species  are three.
are three.
c)
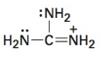
Interpretation:
To draw the maximum resonance structures possible for the species

Concept introduction:
Resonance forms differ only in the placement of their π and nonbonding valence electrons. Neither the position nor the hybridization of any atom changes from one resonance form to another. For writing different resonance forms in the structure given, first a three atom groupings with a multiple bond and a p orbital with pair of electrons is to be identified. Then the exchange of position of double bond and electrons in p orbital will give another resonance form. The shift is represented by a curved arrow.
To draw:
The maximum resonance structures possible for the species

Answer to Problem 38AP
The maximum resonance structures possible for the species  is three.
is three.
Explanation of Solution
Resonance forms differ only in the placement of their π and their nonbonding valence electrons. Neither the position nor the hybridization of any atom changes from one resonance form to another. The cation given has a carbon doubly bonded to positively charged nitrogen and singly bonded to two more nitrogens each with a pair of nonbonding electrons. Using the nonbonding electrons on the nitrogens and the π bond in C=N, two more structures, as shown, can be drawn without change in position or hybridization of any atom. Hence the species given has three resonance forms.
The maximum resonance structures possible for the species  are three.
are three.
d)
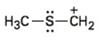
Interpretation:
To draw the maximum resonance structures possible for the species
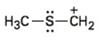
Concept introduction:
Resonance forms differ only in the placement of their π and nonbonding valence electrons. Neither the position nor the hybridization of any atom changes from one resonance form to another. For writing different resonance forms in the structure given, first a three atom groupings with a multiple bond and a p orbital with pair of electrons is to be identified. Then the exchange of position of double bond and electrons in p orbital will give another resonance form. The shift is represented by a curved arrow.
To draw:
The maximum resonance structures possible for the species
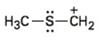
Answer to Problem 38AP
The maximum resonance structures possible for the species 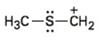 is two
is two
Explanation of Solution
Resonance forms differ only in the placement of their π and their nonbonding valence electrons. Neither the position nor the hybridization of any atom changes from one resonance form to another. The cation given has a carbon atom singly bonded to a sulfur atom which has two lone pairs of electrons. Using the nonbonding electrons on the sulfur atom one more structure can be drawn, as shown, without change in position or hybridization of any atom. Hence the species given has two resonance forms.
The maximum resonance structures possible for the species 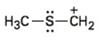 are two.
are two.
e)
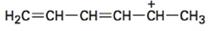
Interpretation:
To draw the maximum resonance structures possible for the species
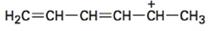
Concept introduction:
Resonance forms differ only in the placement of their π and nonbonding valence electrons. Neither the position nor the hybridization of any atom changes from one resonance form to another. For writing different resonance forms in the structure given, first a three atom groupings with a multiple bond and a p orbital with pair of electrons is to be identified. Then the exchange of position of double bond and electrons in p orbital will give another resonance form. The shift is represented by a curved arrow.
To draw:
The maximum resonance structures possible for the species
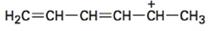
Answer to Problem 38AP
The maximum resonance structures possible for the species 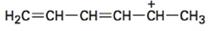 is three.
is three.
Explanation of Solution
Resonance forms differ only in the placement of their π and their nonbonding valence electrons. Neither the position nor the hybridization of any atom changes from one resonance form to another. In the cation given, the positively charged carbon is attached a carbon chain that contains two conjugated double bonds. Using the two π bonds two more structures can be drawn, as shown, without change in position or hybridization of any atom. Hence the species given has three resonance forms.
The maximum resonance structures possible for the species 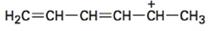 are three.
are three.
Want to see more full solutions like this?
Chapter 2 Solutions
Organic Chemistry
- Draw the missing intermediate 1 and final product 2 of this synthesis: 1. MeO- H3O+ 1 2 2. PrBr Δ You can draw the two structures in any arrangement you like. Click and drag to start drawing a structure.arrow_forwardWhat is the differences between: Glyceride and phosphoglyceride Wax and Fat Soap and Fatty acid HDL and LDL cholesterol Phospho lipids and sphingosine What are the types of lipids? What are the main lipid components of membrane structures? How could lipids play important rules as signaling molecules and building units? The structure variety of lipids makes them to play significant rules in our body, conclude breifly on this statement.arrow_forwardWhat is the differences between DNA and RNA for the following: - structure - function - type What is the meaning of: - replication - transcription - translation show the base pair connection(hydrogen bond) in DNA and RNAarrow_forward
- What is the IP for a amino acid- give an example what are the types of amino acids What are the structures of proteins The N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Try-Val-His Sar-Arg-Val His-Pro-Ala Val- Tyr- Val Arg-Val-Tyr What is the structure of saralasin?arrow_forwardWhat is the IP for a amino acid- give an example what are the types of amino acids What are the structures of proteins The N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Try-Val-His Sar-Arg-Val His-Pro-Ala Val- Tyr- Val Arg-Val-Tyr What is the structure of saralasin?arrow_forward> aw the missing intermediates 1 and 2, plus the final product 3, of this synthesis: 1. Eto 1. EtO¯ H3O+ 1 2 2. PrBr 2. PrBr Δ You can draw the three structures in any arrangement you like. 3 Click and drag to start drawing a structure. Explanation Check 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacarrow_forward
- There are various factors that affect an equilibrium. Give 3 of these factors and explain using examples andequations how an equilibrium is affected by these factors. Please remember that this is a communication question so that you are communicating your understanding of the factors that affect and equilibrium.arrow_forwardEEZE LETCHUP ID Draw the most likely conjugate base resulting from this acid-base reaction. Include all lone pairs. Ignore inorganic byproducts. Drawing く NaOCH2CH3 :0: :0: 狗arrow_forwardAnswerarrow_forward
- 2. Provide a clear arrow-pushing mechanism for the following reactions. Do not skip proton transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted without ambiguity. a. CH3 Ph OEt هد Ph CH3 Hint: the species on the left is an ynolate, which behaves a lot like an enolate.arrow_forwardb. CH3 H3C CH3 CH3 H3C an unexpected product, containing a single 9- membered ring the expected product, containing two fused rings H3C-I (H3C)2CuLi an enolatearrow_forwardb. H3C CH3 1. 2. H3O+ H3C MgBr H3Carrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

