
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2.SE, Problem 27MP
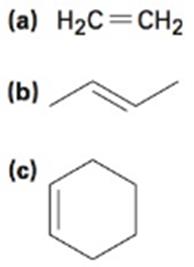
Double bonds can also act like Lewis bases, sharing their electrons with Lewis acids. Use curved arrows to show how each double bond below will react with HCl and draw the resulting carbocation.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Convert the following Fischer projection to Haworth projections. show work and show the arrows please.
Draw the mechanism for the acid-catalyzed dehydration of 2-methyl-hexan-2-ol.
Draw the product of the reaction no mechanism required.
Chapter 2 Solutions
Organic Chemistry
Ch. 2.1 - Prob. 1PCh. 2.1 - Prob. 2PCh. 2.1 - Use the electronegativity values shown in Figure...Ch. 2.1 - Look at the following electrostatic potential map...Ch. 2.2 - Ethylene glycol, HOCH2CH2OH, may look nonpolar...Ch. 2.2 - Make three-dimensional drawings of the following...Ch. 2.3 - Calculate formal charges for the nonhydrogen atoms...Ch. 2.3 - Organic phosphate groups occur commonly in...Ch. 2.6 - Which of the following pairs of structures...Ch. 2.6 - Draw the indicated number of resonance forms for...
Ch. 2.7 - Nitric acid (HNO3) reacts with ammonia (NH3) to...Ch. 2.8 - Prob. 12PCh. 2.8 - Amide ion, H2N-, is a much stronger base than...Ch. 2.9 - Prob. 14PCh. 2.9 - Prob. 15PCh. 2.9 - Prob. 16PCh. 2.11 - Using curved arrows, show how the species in part...Ch. 2.11 - Prob. 18PCh. 2.12 - Of the two vitamins A and C, one is hydrophilic...Ch. 2.SE - Prob. 20VCCh. 2.SE - The following model is a representation of...Ch. 2.SE - cis-l, 2-Dichloroethylene and trans-1,...Ch. 2.SE - The following molecular models are representations...Ch. 2.SE - Predict the product(s) of the acid/base reactions...Ch. 2.SE - Use curved arrows to draw the protonated form of...Ch. 2.SE - Prob. 26MPCh. 2.SE - Double bonds can also act like Lewis bases,...Ch. 2.SE - Prob. 28APCh. 2.SE - Use the electronegativity table given in Figure...Ch. 2.SE - Which of the following molecules has a dipole...Ch. 2.SE - Prob. 31APCh. 2.SE - Phosgene, C12C=O, has a smaller dipole moment than...Ch. 2.SE - Prob. 33APCh. 2.SE - Methanethiol, CH3SH, has a substantial dipole...Ch. 2.SE - Calculate the formal charges on the atoms shown in...Ch. 2.SE - Assign formal charges to the atoms in each of the...Ch. 2.SE - Which of the following pairs of structures...Ch. 2.SE - Prob. 38APCh. 2.SE - 1, 3-Cyclobutadiene is a rectangular molecule with...Ch. 2.SE - Alcohols can act either as weak acids or as weak...Ch. 2.SE - The O-H hydrogen in acetic acid is more acidic...Ch. 2.SE - Draw electron-dot structures for the following...Ch. 2.SE - Write the products of the following acid-base...Ch. 2.SE - Rank the following substances in order of...Ch. 2.SE - Which, if any, of the substances in Problem 2-44...Ch. 2.SE - The ammonium ion (NH4+, pKa = 9.25) has a lower...Ch. 2.SE - Prob. 47APCh. 2.SE - Prob. 48APCh. 2.SE - Calculate Ka values from the following pka’s:...Ch. 2.SE - Calculate pKa values from the following Ka’s:...Ch. 2.SE - What is the pH of a 0.050 M solution of formic...Ch. 2.SE - Prob. 52APCh. 2.SE - Maleic acid has a dipole moment, but the closely...Ch. 2.SE - Assume that you have two unlabeled bottles, one of...Ch. 2.SE - Identify the acids and bases in the following...Ch. 2.SE - Which of the following pairs represent resonance...Ch. 2.SE - Draw as many resonance structures as you can for...Ch. 2.SE - Carbocations, which contain a trivalent,...Ch. 2.SE - We’ll see in the next chapter that organic...Ch. 2.SE - The azide functional group, which occurs in...Ch. 2.SE - Phenol, C6H5OH, is a stronger acid than methanol,...Ch. 2.SE - Thiamin diphosphate (TPP), a derivative of vitamin...Ch. 2.SE - Determine if each compound or ion below has a...Ch. 2.SE - Prob. 64APCh. 2.SE - Prob. 65APCh. 2.SE - Draw the conjugate base for each compound below...Ch. 2.SE - 1, 1, 1-Trichloroethanol is an acid more than 1000...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Name the following carbohydrates give both the systematic and common names. Don't forget to identify the Isomer.arrow_forwardWhat is the product of the reaction of XeF4 with H2O? Group of answer choices H2XeF2 H2XeF4 XeO3 H2XeOarrow_forwardWhile noble gas exerts the strongest London (dispersion) forces on neighboring atoms? Group of answer choices Xe Ar Kr Nearrow_forward
- Which of the following elements is corrosive to your skin due to that element breaking down C=C bonds? Group of answer choices fluorine iodine bromine chlorinearrow_forwardWhat the best source of sulfide to use on a small scale in the lab? Group of answer choices thiourea H2S NaHS Na2Sarrow_forwardWhich of the following statements about sulfur is FALSE? Group of answer choices H2S is the product of an oxygen-depleted ecosystem. In the acid mine drainage reaction, FeS2 is a product. One allotrope of sulfur has the formula S20. In the environment, bacterial oxidation can convert S2− to elemental S or SO42−.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Mass Spectrometry; Author: Professor Dave Explains;https://www.youtube.com/watch?v=hSirWciIvSg;License: Standard YouTube License, CC-BY