
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2.SE, Problem 62AP
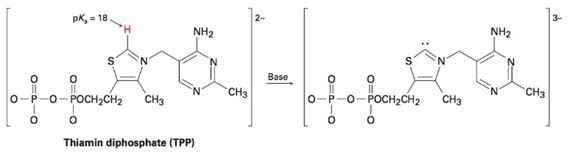
Thiamin diphosphate (TPP), a derivative of vitamin B1 required for glucose
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Is the molecule chiral, meso, or achiral?
H3C
H3C-
Br
Br
Identify the chiral carbon as R- or S-.
CH3
Identify priority of the substituents:
Br- H
Chapter 2 Solutions
Organic Chemistry
Ch. 2.1 - Prob. 1PCh. 2.1 - Prob. 2PCh. 2.1 - Use the electronegativity values shown in Figure...Ch. 2.1 - Look at the following electrostatic potential map...Ch. 2.2 - Ethylene glycol, HOCH2CH2OH, may look nonpolar...Ch. 2.2 - Make three-dimensional drawings of the following...Ch. 2.3 - Calculate formal charges for the nonhydrogen atoms...Ch. 2.3 - Organic phosphate groups occur commonly in...Ch. 2.6 - Which of the following pairs of structures...Ch. 2.6 - Draw the indicated number of resonance forms for...
Ch. 2.7 - Nitric acid (HNO3) reacts with ammonia (NH3) to...Ch. 2.8 - Prob. 12PCh. 2.8 - Amide ion, H2N-, is a much stronger base than...Ch. 2.9 - Prob. 14PCh. 2.9 - Prob. 15PCh. 2.9 - Prob. 16PCh. 2.11 - Using curved arrows, show how the species in part...Ch. 2.11 - Prob. 18PCh. 2.12 - Of the two vitamins A and C, one is hydrophilic...Ch. 2.SE - Prob. 20VCCh. 2.SE - The following model is a representation of...Ch. 2.SE - cis-l, 2-Dichloroethylene and trans-1,...Ch. 2.SE - The following molecular models are representations...Ch. 2.SE - Predict the product(s) of the acid/base reactions...Ch. 2.SE - Use curved arrows to draw the protonated form of...Ch. 2.SE - Prob. 26MPCh. 2.SE - Double bonds can also act like Lewis bases,...Ch. 2.SE - Prob. 28APCh. 2.SE - Use the electronegativity table given in Figure...Ch. 2.SE - Which of the following molecules has a dipole...Ch. 2.SE - Prob. 31APCh. 2.SE - Phosgene, C12C=O, has a smaller dipole moment than...Ch. 2.SE - Prob. 33APCh. 2.SE - Methanethiol, CH3SH, has a substantial dipole...Ch. 2.SE - Calculate the formal charges on the atoms shown in...Ch. 2.SE - Assign formal charges to the atoms in each of the...Ch. 2.SE - Which of the following pairs of structures...Ch. 2.SE - Prob. 38APCh. 2.SE - 1, 3-Cyclobutadiene is a rectangular molecule with...Ch. 2.SE - Alcohols can act either as weak acids or as weak...Ch. 2.SE - The O-H hydrogen in acetic acid is more acidic...Ch. 2.SE - Draw electron-dot structures for the following...Ch. 2.SE - Write the products of the following acid-base...Ch. 2.SE - Rank the following substances in order of...Ch. 2.SE - Which, if any, of the substances in Problem 2-44...Ch. 2.SE - The ammonium ion (NH4+, pKa = 9.25) has a lower...Ch. 2.SE - Prob. 47APCh. 2.SE - Prob. 48APCh. 2.SE - Calculate Ka values from the following pka’s:...Ch. 2.SE - Calculate pKa values from the following Ka’s:...Ch. 2.SE - What is the pH of a 0.050 M solution of formic...Ch. 2.SE - Prob. 52APCh. 2.SE - Maleic acid has a dipole moment, but the closely...Ch. 2.SE - Assume that you have two unlabeled bottles, one of...Ch. 2.SE - Identify the acids and bases in the following...Ch. 2.SE - Which of the following pairs represent resonance...Ch. 2.SE - Draw as many resonance structures as you can for...Ch. 2.SE - Carbocations, which contain a trivalent,...Ch. 2.SE - We’ll see in the next chapter that organic...Ch. 2.SE - The azide functional group, which occurs in...Ch. 2.SE - Phenol, C6H5OH, is a stronger acid than methanol,...Ch. 2.SE - Thiamin diphosphate (TPP), a derivative of vitamin...Ch. 2.SE - Determine if each compound or ion below has a...Ch. 2.SE - Prob. 64APCh. 2.SE - Prob. 65APCh. 2.SE - Draw the conjugate base for each compound below...Ch. 2.SE - 1, 1, 1-Trichloroethanol is an acid more than 1000...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- I need the nomenclature of this compoundarrow_forward2. Name the following hydrocarbons. (9 marks) a) HHHHHHHH H-C-C- H-O-S b) HCEC-CH3 H H H H H d) c) H C=C- H H H e) CH3 CH3 CH2CH=CH-CH=CHCH3 HHHH H-C-C-C-C-H H HH H f) large CH2CH3 pola H3C section lovels tower, able ocart firs g) Tower H3C-CH2 then in H3C-CH-CH-CH3 enblbano bne noitsidab Copyright © 2008. Durham Continuing Education CH3arrow_forwardName the molecules & Identify any chiral center CH3CH2CH2CHCH₂CH₂CH₂CH₂ OH CH₂CHCH2CH3 Br CH3 CH3CHCH2CHCH2CH3 CH3arrow_forward
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).arrow_forwardWhat is the IUPAC name of the following compound? CH₂CH₂ H CI H₂CH₂C H CH₂ Selected Answer: O (35,4R)-4 chloro-3-ethylpentane Correctarrow_forward
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. I I I H Select to Add Arrows HCI, CH3CH2OHarrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and the follow the arrows to draw the intermediate and product in this reaction or mechanistic step(s).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY