
Concept explainers
Interpretation:
The condensed structural formulaof ethane needs to be drawn.
Concept introduction:
The hydrocarbon compounds that contains single bond(s) between carbon atom(s) are said to be saturated hydrocarbon. Hydrocarbon compounds containing single bonds only are said to be
Answer to Problem 51A
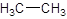
Explanation of Solution
Alkanes are compounds containing carbon and hydrogen atoms having single bond(s) between carbon atom(s) in the structure. For number of carbons atoms in alkane chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
The given name is ethane that means the parent chain has 2 number of carbons in the chain and there is no substituent in the chain. So, the condensed structural formulaof ethane is:
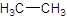
Interpretation:
The condensed structural formulaof hexane needs to be drawn.
Concept introduction:
The hydrocarbon compounds that contains single bond(s) between carbon atom(s) are said to be saturated hydrocarbon. Hydrocarbon compounds containing single bonds only are said to be alkane.
Answer to Problem 51A
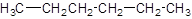
Explanation of Solution
The given name is hexane that means the parent chain has 6 number of carbons in the chain and there is no substituent in the chain. So, the condensed structural formulaof hexane is:
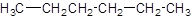
Interpretation:
The condensed structural formulaof propane needs to be drawn.
Concept introduction:
The hydrocarbon compounds that contains single bond(s) between carbon atom(s) are said to be saturated hydrocarbon. Hydrocarbon compounds containing single bonds only are said to be alkane.
Answer to Problem 51A
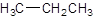
Explanation of Solution
The given name is propane that means the parent chain has 3 number of carbons in the chain and there is no substituent in the chain. So, the condensed structural formulaof propane is:
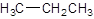
Interpretation:
The condensed structural formulaof heptane needs to be drawn.
Concept introduction:
The hydrocarbon compounds that contains single bond(s) between carbon atom(s) are said to be saturated hydrocarbon. Hydrocarbon compounds containing single bonds only are said to be alkane.
Answer to Problem 51A
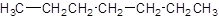
Explanation of Solution
The given name is heptane that means the parent chain has 7 number of carbons in the chain and there is no substituent in the chain. So, the condensed structural formulaof heptane is:
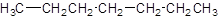
Chapter 21 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Biology: Life on Earth (11th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Campbell Biology in Focus (2nd Edition)
Human Physiology: An Integrated Approach (8th Edition)
Introductory Chemistry (6th Edition)
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
- Show how to convert ethyl benzene to (a) 2,5-dichlorobenzoic acid and (b) 2,4-dichlorobenzoic acid.arrow_forwardno aiarrow_forwardPolymers may be composed of thousands of monomers. Draw three repeat units (trimer) of the polymer formed in this reaction. Assume there are hydrogen atoms there are hydrogen atoms on the two ends of the trimer. Ignore inorganic byproducts.arrow_forward
- 8:44 PM Sun Apr 13 Earn Freecash.com O Measurement and Matter =1 Setting up a unit conversion 110 Eddie says... ✰ www-awu.aleks.com A student sets up the following equation to convert a measurement. (The ? stands for a number the student is going to calculate.) Fill in the missing part of this equation. Note: your answer should be in the form of one or more fractions multiplied together. (- 4 J kJ -7.0 × 10 ☐ = ? mmol.°C mol °C x10 μ Explanation Check □·□ torox.io Grey Hill LLC. All Rightsarrow_forwardPolymers may be composed of thousands of monomers. Draw three repeat units (trimer) of the polymer formed in this reaction. Assume there are hydrogen atoms there are hydrogen atoms on the two ends of the trimer. Ignore inorganic byproducts please.arrow_forwardi need help with the folarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





