
Concept explainers
Interpretation:
The changes occurs in molecular arrangement which takes place when the pressure is increased from 8 atm to 16 atm and the temperature remains constant at 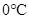 should be described.
should be described.
Concept introduction:
When energy or heat is gain or lost by any matter, the matter changes its state to form a
State of matter is classified as:
- Solid
- Liquid
- Gas
A graphical diagram which represents the physical states of a substance under different values of temperature and pressure is known as phase diagram.
The general representation of phase diagram is:
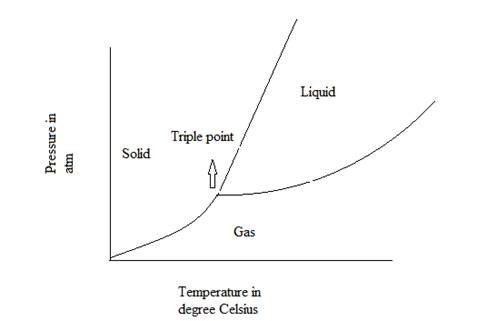
Triple point of a substance is defined as the point at which solid, liquid and gas phase or state of that substance coexist in the equilibrium.
Answer to Problem 12STP
A substance changesfrom gas phase to liquid phase when the pressure is increased from 8 atm to 16 atm and the temperature remains constant at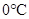
Explanation of Solution
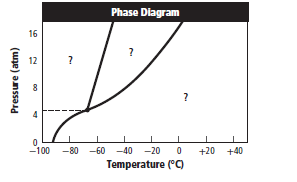
The given graph is:
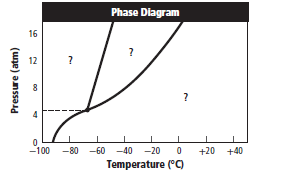
Graph can be redrawn as:
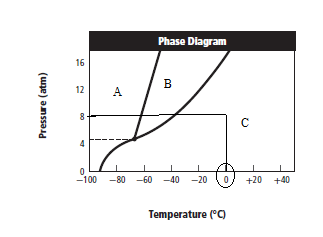
From above graph and general graph of phase diagram, at pressure 8 atm and temperature of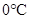 the labeled area (C) represents gas phase.
the labeled area (C) represents gas phase.
When the pressure is increased to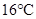 change in state occurs.
change in state occurs.
Graph can be drawn as:
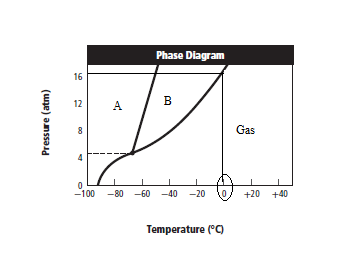
In above graph, one can see that the when the pressure is 16 atm, then by drawing straight line or parallel line to y-axis, substance crosses the line into liquid state.
Thus, when the pressure is increased from 8 atm to 16 atm and the temperature remains constant at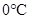 then a substance changes from gas state to liquid state.
then a substance changes from gas state to liquid state.
Chapter 21 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Human Physiology: An Integrated Approach (8th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Biology: Life on Earth (11th Edition)
Campbell Essential Biology (7th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Concepts of Genetics (12th Edition)
- Can I get help on drawing my arrowsarrow_forwardCan I get helpp drawing my arrowsarrow_forwardWhich of the m/z values corresponds to the base peak in the mass spectrum shown? 100 80 A. 45 B. 44 C. 29 D. 15 Intensity 20 0 10 20 30 40 B- m/z -8 50 E. 30 Which of the m/z values correspond to the molecular ion for the compound shown? A. 18 B. 82 OH C. 100 D. 102 E. 103arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





