
Interpretation:
The IUPAC name of the following compound should be determined:
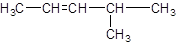
Concept introduction:
The hydrocarbon compounds contain compound that are made up of only hydrogen and carbon atoms. Hydrocarbon compounds that contains multiple bond(s) are said to be
Answer to Problem 17PP
The IUPAC name of the compound is4-methylpent-2-ene.
Explanation of Solution
In order to give the name to the unsaturated hydrocarbon, alkene following steps are followed:
1. The parent (longest) continuous carbon chain containing multiple bonds between the carbon atoms is selected.
2. While writing the name of alkene, the suffix “ane” of the corresponding
3. Name should be written in alphabetical order and numbering should be done in such a way that the multiple bond and substituent group gets lowest number.
4. Hyphen is used to connect the number to the name.
For number of carbons atoms in alkane chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
The given structure is:
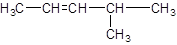
The parent chain in the given structure is of 5-carbon atoms so, prefix will be pent. Numbering is done in such a way that the multiple bond that is double bond gets lower number that is 2. A methyl substituent is present at position 4.
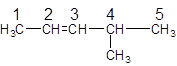
So, the IUPAC name will be: 4-methylpent-2-ene.
Interpretation:
The IUPAC name of the following compound should be determined:
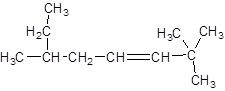
Concept introduction:
The hydrocarbon compounds contain compound that are made up of only hydrogen and carbon atoms. Hydrocarbon compounds that contains multiple bond(s) are said to be unsaturated hydrocarbon. Compounds containing double bonds are said to be alkene whereas compounds containing triple bonds are said to be alkyne.
Answer to Problem 17PP
The IUPAC name of the compound is2, 2, 6-trimethyloct-3-ene.
Explanation of Solution
The given structure is:
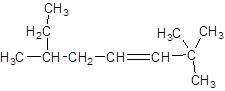
The parent chain in the given structure is of 8-carbon atoms so, prefix will be oct. Numbering is done in such a way that the multiple bond that is double bond gets lower number that is 3. Three methyl substituentsare present at position two at 2 and 6.
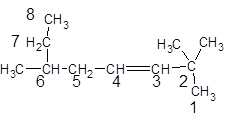
So, the IUPAC name will be: 2, 2, 6-trimethyloct-3-ene.
Chapter 21 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Human Physiology: An Integrated Approach (8th Edition)
Microbiology with Diseases by Body System (5th Edition)
Anatomy & Physiology (6th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Human Anatomy & Physiology (2nd Edition)
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
- A mixture of C7H12O2, C9H9OCl, biphenyl and acetone was put together in a gas chromatography tube. Please decide from the GC resutls which correspond to the peak for C7,C9 and biphenyl and explain the reasoning based on GC results. Eliminate unnecessary peaks from Gas Chromatography results.arrow_forwardIs the molecule chiral, meso, or achiral? CI .CH3 H₂C CIarrow_forwardPLEASE HELP ! URGENT!arrow_forward
- Identify priority of the substituents: CH3arrow_forwardHow many chiral carbons are in the molecule? OH F CI Brarrow_forwardA mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





