
Interpretation:
The values of k should be calculated for the reaction.
Concept introduction:
Rate Law can be expressed as an integrated rate law and a differential rate law.
Differential Rate Law: This describes the change in the concentrations of reactant as a function of time.
Integrated Rate Law: This describes the initial concentrations and the measured concentration of one or more reactants as a function of time.
The proportionality coefficient which relates the
Answer to Problem 14STP
The value of rate constant is equal to
Explanation of Solution
Given information:
Data is given as:
| Initial rate ( |
||
| 0.10 M | 0.10 M | |
| 0.30 M | 0.10 M | |
| 0.30 M | 0.20 M |
The general expression of rate law is expressed as:
Where, m and n are the experimentally determined values.
Experiment 1:
Rate law expression is written as:
Experiment 2:
Rate law expression is written as:
Experiment 3:
Rate law expression is written as:
Now, divide equation (2) by (1):
Now, divide equation (3) by (2):
Thus, rate law expression is written as:
Now,
For experiment 1:
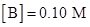
Rate = 7.93 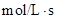
Put the values, in rate law expression:
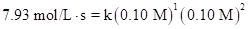
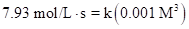
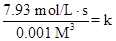
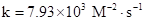
Thus, value of rate constant is equal to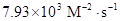
Chapter 21 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Human Anatomy & Physiology (2nd Edition)
Campbell Biology: Concepts & Connections (9th Edition)
Cosmic Perspective Fundamentals
Anatomy & Physiology (6th Edition)
Microbiology: An Introduction
Campbell Biology in Focus (2nd Edition)
- Can I get help on drawing my arrowsarrow_forwardCan I get helpp drawing my arrowsarrow_forwardWhich of the m/z values corresponds to the base peak in the mass spectrum shown? 100 80 A. 45 B. 44 C. 29 D. 15 Intensity 20 0 10 20 30 40 B- m/z -8 50 E. 30 Which of the m/z values correspond to the molecular ion for the compound shown? A. 18 B. 82 OH C. 100 D. 102 E. 103arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





