
Concept explainers
For each amino acid: [1] draw the L enantiomer in a Fischer projection; [2] classify the amino acid as neutral, acidic, or basic; [3] give the three-letter symbol; [4] give the one-letter symbol.
- leucine
- tryptophan
- lysine
- aspartic acid
(a)
Interpretation:
The L-isomer of leucine in Fisher projection needs to be drawn and should be identified as acidic, basic or neutral. The three letter and one symbol for it needs to be determined.
Concept Introduction:
Like monosaccharides, the prefixes D and L are used to designate the arrangement of groups to the chiral center of amino acid, when drawn with a vertical carbon chain having
Amino acids that have the
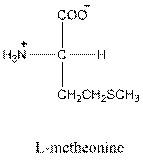
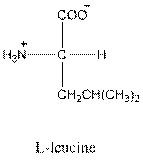
Answer to Problem 25P
- L-isomer of leucine is as follows:
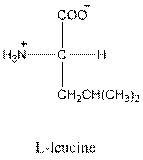
- Leucine is neutral.
- Three-letter symbol for leucine is Leu.
- One-letter symbol for leucine is L.
Explanation of Solution
For leucine the R group is
The amino acid leucine has one positive charge on amino group
One letter symbol for leucine is L and three-letter symbol for leucine is Leu.
(b)
Interpretation:
To draw L-isomer of tryptophan (amino acid) in Fisher projection, to identify it is acidic, basic or neutral and to give three letter and one symbol for it
Concept Introduction : :
Like monosaccharides, the prefixes D and L are used to designate the arrangement of groups to the chiral center of amino acid, when drawn with a vertical carbon chain having
Amino acids that have the
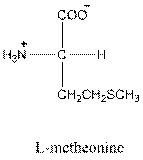
Answer to Problem 25P
- L-isomer of tryptophan is as follows:
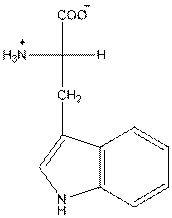
- Amino acid tryptophan is neutral.
Explanation of Solution
For tryptophan the R group is as follows:
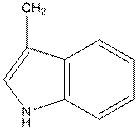
And in L-isomer the amino group
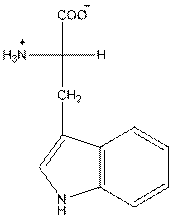
The amino acid tryptophan has one positive charge on amino group
One letter symbol for tryptophan is W and three-letter symbol for tryptophan is Trp.
(c)
Interpretation:
The L-isomer of lysine in Fisher projection needs to be drawn, whether it is acidic, basic or neutral needs to be determined and the three-letter and one symbol for it needs to be given.
Concept Introduction:
Like monosaccharides, the prefixes D and L are used to designate the arrangement of groups to the chiral center of amino acid, when drawn with a vertical carbon chain having
Amino acids that have the
Answer to Problem 25P
- L-isomer of lysine is as follows:
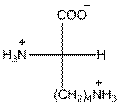
- The amino acid lysine is basic in nature.
- The three letter symbol for lysine is Lys.
- The one letter symbol for lysine is K.
Explanation of Solution
For lysine the R group is
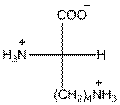
The amino acid lysine has one positive charge on amino group
The three letter symbol for lysine is Lys and one letter symbol for lysine is K.
(d)
Interpretation:
The L-isomer of aspartic acid in Fisher projection needs to be drawn, whether it is acidic, basic or neutral needs to be determined and the three-letter and one symbol for it needs to be given.
Concept Introduction:
Like monosaccharides, the prefixes D and L are used to designate the arrangement of groups to the chiral center of amino acid, when drawn with a vertical carbon chain having
Amino acids that have the
Answer to Problem 25P
- L-isomer of aspartic acid is as follows:
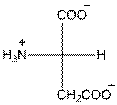
- The aspartic acid is acidic in nature.
- The three letter symbol for aspartic acid is Asp.
- The one letter symbol for aspartic acid is D.
Explanation of Solution
For aspartic acid the R group is
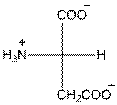
The aspartic acid has one positive charge on amino group
The three letter symbol for aspartic acid is Asp and the one letter symbol for aspartic acid is D.
Want to see more full solutions like this?
Chapter 21 Solutions
General, Organic, and Biological Chemistry - 4th edition
- Provide the IUPAC name of the following molecule. Don't forget to include the proper stereochemistry where appropriate.arrow_forward3. 2. 1. On the graph below, plot the volume of rain in milliliters versus its height in centimeters for the 400 mL beaker. Draw a straight line through the points and label it "400 mL beaker." Volume (mL) 400 350 300 250 200 150 750 mL Florence Volume Versus Height of Water 400 mL beaker 100 50 0 0 2 3 4 5 Height (cm) 6 7 8 9 10 Explain why the data points for the beaker lie roughly on a straight line. What kind of relationship is this? How do you know? (see page 276 text) the design of the beaker is a uniform cylinder the volume of liquid increases evenly with its height resulting in a linear relationship. What volume would you predict for 10.0 cm of water? Explain how you arrived at your answer. Use the data table and the graph to assist you in answering the question. 4. Plot the volume of rain in milliliters versus its height in centimeters for the 250 mL Florence flask on the same graph. Draw a best-fit curve through the points and label it "250 mL Florence flask." oke camearrow_forwardShow work. Don't give Ai generated solutionarrow_forward
- In the video, we looked at the absorbance of a certain substance and how it varies depending on what wavelength of light we are looking at. Below is a similar scan of a different substance. What color BEST describes how this substance will appear? Absorbance (AU) Violet Blue Green Orange 1.2 1.0- 0.8- 0.6- 0.4- 0.2 0.0 450 500 550 600 650 700 Wavelength (nm) violet indigo blue green yellow orange red Red O Cannot tell from this information In the above graph, what causes -450 nm wavelength of light to have a higher absorbance than light with a -550 nm wavelength? Check all that are true. The distance the light travels is different The different data points are for different substances The concentration is different at different times in the experiment Epsilon (molar absortivity) is different at different wavelengthsarrow_forward5. a. Data were collected for Trial 1 to determine the molar mass of a nonvolatile solid solute when dissolved in cyclo- hexane. Complete the table for the analysis (See Report Sheet). Record calculated values with the correct number of significant figures. B. Freezing Point of Cyclohexane plus Calculation Zone Unknown Solute 2. Mass of cyclohexane (g) 10.14 Part C.4 3. Mass of added solute (g) 0.255 C. Calculations 1. k; for cyclohexane (°C⚫ kg/mol) 20.0 2. Freezing point change, AT, (°C) 3.04 Part C.6 3. Mass of cyclohexane in solution (kg) 4. Moles of solute, total (mol) Show calculation. 5. Mass of solute in solution, total (g) 6. Molar mass of solute (g/mol) Show calculation.arrow_forwardDraw and name the R groups of all 20 amino acids.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





