
EBK INTRO.CHEMISTRY (NASTA EDITION)
9th Edition
ISBN: 9781337678032
Author: ZUMDAHL
Publisher: CENGAGE CO
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 20, Problem 71QAP
Interpretation Introduction
Interpretation:
The structures of the respective
Concept Introduction:
Aldehyde and ketone are considered to be two main
Aldehyde functional group:
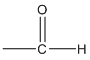
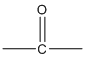
Ketone functional group:
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
These are in the wrong boxes. Why does the one on the left have a lower molar mass than the one on the right?
SYNTHESIS REACTIONS. For the following reactions, synthesize the given products from the given reactants.
Multiple reactions/steps will be needed. For the one of the steps (ie reactions) in each synthesis, write out the
mechanism for that reaction and draw an energy diagram showing the correct number of hills and valleys for
that step's mechanism.
CI
b.
a.
Use acetylene (ethyne)
and any alkyl halide as
your starting materials
Br
C.
d.
"OH
OH
III.
OH
Calculate the pH and the pOH of each of the following solutions at 25 °C for which the substances ionize completely:
(a) 0.200 M HCl
Chapter 20 Solutions
EBK INTRO.CHEMISTRY (NASTA EDITION)
Ch. 20.2 - Exercise 20.1 Give the molecular formulas for the...Ch. 20.4 - Exercise 20.2 Name the following molecules. a. b.Ch. 20.4 - Exercise 20.3 Write the structural formula for...Ch. 20.5 - Petroleum is a very valuable raw material for the...Ch. 20.7 - Exercise 20.4 Name the following molecules. a. b.Ch. 20.9 - Prob. 20.5SCCh. 20.11 - Prob. 20.6SCCh. 20.14 - Prob. 20.7SCCh. 20 - What is meant by the term “unsaturated...Ch. 20 - Prob. 2ALQ
Ch. 20 - Prob. 3ALQCh. 20 - How many different possible “tetramethylbenzenes”...Ch. 20 - For the general formula C6H14O, draw the...Ch. 20 - Prob. 6ALQCh. 20 - Prob. 1QAPCh. 20 - Your roommate, a chemistry major, claims to have...Ch. 20 - Prob. 3QAPCh. 20 - How many electron pairs are shared when a triple...Ch. 20 - Prob. 5QAPCh. 20 - Prob. 6QAPCh. 20 - Prob. 7QAPCh. 20 - Prob. 8QAPCh. 20 - Prob. 9QAPCh. 20 - . The chains in normal alkanes are not really...Ch. 20 - Prob. 11QAPCh. 20 - Prob. 12QAPCh. 20 - . Give the name of each of the following...Ch. 20 - Prob. 14QAPCh. 20 - . What are structural isomers? Which is the...Ch. 20 - Prob. 16QAPCh. 20 - Prob. 17QAPCh. 20 - Prob. 18QAPCh. 20 - Prob. 19QAPCh. 20 - Prob. 20QAPCh. 20 - . What is an alkyl group? How is a given alkyl...Ch. 20 - . When naming alkanes, find the longest continuous...Ch. 20 - Prob. 23QAPCh. 20 - . When naming alkanes, the alkyl groups are listed...Ch. 20 - . Give the systematic name for each of the...Ch. 20 - . Give the systematic name for each of the...Ch. 20 - Prob. 27QAPCh. 20 - Prob. 28QAPCh. 20 - Prob. 29QAPCh. 20 - Prob. 30QAPCh. 20 - . What is pyrolytic cracking, and why is the...Ch. 20 - Prob. 32QAPCh. 20 - . Explain why alkanes are relatively unreactive.Ch. 20 - Prob. 34QAPCh. 20 - . Indicate the missing molecule in each of the...Ch. 20 - Prob. 36QAPCh. 20 - Prob. 37QAPCh. 20 - Prob. 38QAPCh. 20 - Prob. 39QAPCh. 20 - Prob. 40QAPCh. 20 - Prob. 41QAPCh. 20 - Prob. 42QAPCh. 20 - Prob. 43QAPCh. 20 - Prob. 44QAPCh. 20 - Prob. 45QAPCh. 20 - Prob. 46QAPCh. 20 - Prob. 47QAPCh. 20 - Prob. 48QAPCh. 20 - Prob. 49QAPCh. 20 - . Benzene exhibits resonance Explain this...Ch. 20 - . How is a monosubstituted benzene named? Give the...Ch. 20 - Prob. 52QAPCh. 20 - Prob. 53QAPCh. 20 - . What do the prefixes ortho-, meta-, and para-...Ch. 20 - Prob. 55QAPCh. 20 - Prob. 56QAPCh. 20 - Prob. 57QAPCh. 20 - Prob. 58QAPCh. 20 - . What functional group characterizes an alcohol?...Ch. 20 - Prob. 60QAPCh. 20 - . Give the systematic name for each of the...Ch. 20 - . Is 1-pentanol a primary, secondary, or tertiary...Ch. 20 - . Why is methanol sometimes called wood alcohol?...Ch. 20 - Prob. 64QAPCh. 20 - . Write the equation for the synthesis of ethanol...Ch. 20 - . What is the simplest aromatic alcohol commonly...Ch. 20 - Prob. 67QAPCh. 20 - Prob. 68QAPCh. 20 - Prob. 69QAPCh. 20 - Prob. 70QAPCh. 20 - Prob. 71QAPCh. 20 - Prob. 72QAPCh. 20 - Prob. 73QAPCh. 20 - Prob. 74QAPCh. 20 - Prob. 75QAPCh. 20 - Prob. 76QAPCh. 20 - Prob. 77QAPCh. 20 - Prob. 78QAPCh. 20 - Prob. 79QAPCh. 20 - Prob. 80QAPCh. 20 - Prob. 81QAPCh. 20 - . Draw a structural formula for each of the...Ch. 20 - Prob. 83QAPCh. 20 - Prob. 84QAPCh. 20 - Prob. 85QAPCh. 20 - Prob. 86QAPCh. 20 - Prob. 87QAPCh. 20 - Prob. 88QAPCh. 20 - Prob. 89APCh. 20 - Prob. 90APCh. 20 - Prob. 91APCh. 20 - Prob. 92APCh. 20 - Prob. 93APCh. 20 - . The systematic names of all saturated...Ch. 20 - Prob. 95APCh. 20 - Prob. 96APCh. 20 - Prob. 97APCh. 20 - Prob. 98APCh. 20 - Prob. 99APCh. 20 - . With very reactive agents, such as the halogen...Ch. 20 - . Alkenes and alkynes are characterized by their...Ch. 20 - Prob. 102APCh. 20 - Prob. 103APCh. 20 - Prob. 104APCh. 20 - Prob. 105APCh. 20 - Prob. 106APCh. 20 - Prob. 107APCh. 20 - Prob. 108APCh. 20 - Prob. 109APCh. 20 - Prob. 110APCh. 20 - Prob. 111APCh. 20 - Prob. 112APCh. 20 - Prob. 113APCh. 20 - Prob. 114APCh. 20 - Prob. 115APCh. 20 - . Give the systematic name for each of the...Ch. 20 - Prob. 117APCh. 20 - Prob. 118APCh. 20 - Prob. 119APCh. 20 - Prob. 120APCh. 20 - Prob. 121APCh. 20 - Prob. 122APCh. 20 - Prob. 123APCh. 20 - Prob. 124APCh. 20 - Prob. 125APCh. 20 - Prob. 126APCh. 20 - Prob. 127APCh. 20 - Prob. 128APCh. 20 - Prob. 129APCh. 20 - Prob. 130APCh. 20 - Prob. 131APCh. 20 - . Write the formula for the missing reactant or...Ch. 20 - Prob. 133APCh. 20 - Prob. 134APCh. 20 - . Name each of the following aromatic or...Ch. 20 - Prob. 136APCh. 20 - Prob. 137APCh. 20 - Prob. 138APCh. 20 - Prob. 139APCh. 20 - Prob. 140APCh. 20 - Prob. 141APCh. 20 - . Name each of the following alkanes....Ch. 20 - Prob. 143CPCh. 20 - Prob. 144CPCh. 20 - Prob. 145CPCh. 20 - Prob. 146CPCh. 20 - Prob. 147CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the pH and the pOH of each of the following solutions at 25 °C for which the substances ionize completely: (a) 0.000259 M HClO4arrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardDetermine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. NaN₃arrow_forward
- A. Draw the structure of each of the following alcohols. Then draw and name the product you would expect to produce by the oxidation of each. a. 4-Methyl-2-heptanol b. 3,4-Dimethyl-1-pentanol c. 4-Ethyl-2-heptanol d. 5,7-Dichloro-3-heptanolarrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardCan I please get help with this.arrow_forward
- Determine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forwardPlease help me with identifying these.arrow_forwardCan I please get help with this?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,