
Concept explainers
Write the chemical formula and Lewis structure of the following each of which contains five carbon atoms:
(a) an
(b) an
(c) an
a)
Interpretation:
The chemical formula and Lewis structure of an alkane with five carbon atoms are to be written.
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Hydrocarbons are classified as saturated hydrocarbon and unsaturated hydrocarbon. Saturated hydrocarbons are those hydrocarbons in which carbon-carbon single bond is present as carbon is linked with four atoms. Unsaturated hydrocarbons are those hydrocarbons in which carbon-carbon multiple bonds are present that is double and triple bond.
The completely saturated hydrocarbon is known as an alkane.
The general molecular formula of alkane is
Answer to Problem 1E
Chemical formula:
Lewis structure:
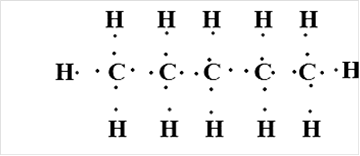
Pentane
Explanation of Solution
The general formula of alkane is
In case of five carbon atoms, the molecular formula of alkane is
- Lewis structures are the diagrams that show the bonding between the atoms of the molecules and existing lone pairs of electrons.
- Bonding electrons are those electrons which are shared between the atoms resulting in the formation of bond.
- Non-bonding electrons are the valence electrons of the atom which are not shared with another atom.
Number of valence electrons in a carbon atom = 4
Number of valence electrons in a hydrogen atom = 1
Total number of valence electrons =
= 32
Thus, Lewis structure of alkane having five carbon atoms is:
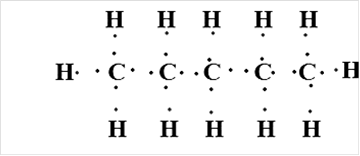 Or,
Or, 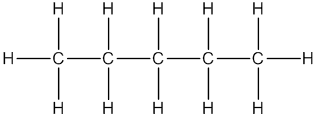
b)
Interpretation:
The chemical formula and Lewis structure of an alkene with five carbon atoms are to be written.
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Hydrocarbons are classified as saturated hydrocarbon and unsaturated hydrocarbon. Saturated hydrocarbons are those hydrocarbons in which carbon-carbon single bond is present as carbon is linked with four atoms. Unsaturated hydrocarbons are those hydrocarbons in which carbon-carbon multiple bonds are present that is double and triple bond.
The unsaturated hydrocarbon with one or more double bond is known as an alkene.
The general molecular formula of alkene is
Answer to Problem 1E
Chemical formula:
Lewis structure:
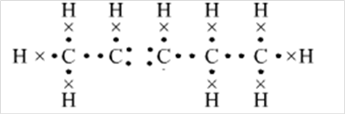
Explanation of Solution
The general formula of alkene is
In case of five carbon atoms, the molecular formula of alkene is
- Lewis structures are the diagrams that show the bonding between the atoms of the molecules and existing lone pairs of electrons.
- Bonding electrons are those electrons which are shared between the atoms resulting in the formation of bond.
- Non-bonding electrons are the valence electrons of the atom which are not shared with another atom.
Number of valence electrons in a carbon atom = 4
Number of valence electrons in a hydrogen atom = 1
Total number of valence electrons =
= 30
Thus, Lewis structure of alkene having five carbon atoms is:
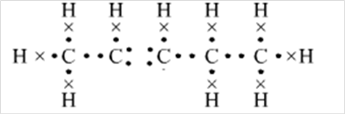 Or,
Or, 
c)
Interpretation:
The chemical formula and Lewis structure of an alkyne with five carbon atoms are to be written.
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Hydrocarbons are classified as saturated hydrocarbon and unsaturated hydrocarbon. Saturated hydrocarbons are those hydrocarbons in which carbon-carbon single bond is present as carbon is linked with four atoms.Unsaturated hydrocarbons are those hydrocarbons in which carbon-carbon multiple bonds are present that is double and triple bond.
The unsaturated hydrocarbon with one or more triple bond is known as an alkyne.
The general molecular formula of alkyne is
Answer to Problem 1E
Chemical formula:
Lewis structure:
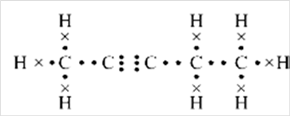
Explanation of Solution
The general formula of alkyne is
In case of five carbon atoms, the molecular formula of alkyne is
- Lewis structures are the diagrams that show the bonding between the atoms of the molecules and existing lone pairs of electrons.
- Bonding electrons are those electrons which are shared between the atoms resulting in the formation of bond.
- Non-bonding electrons are the valence electrons of the atom which are not shared with another atom.
Number of valence electrons in a carbon atom = 4
Number of valence electrons in a hydrogen atom = 1
Total number of valence electrons =
= 28
Thus, Lewis structure of alkyne having five carbon atoms is:
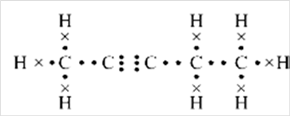 Or,
Or, 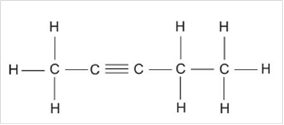
Want to see more full solutions like this?
Chapter 20 Solutions
Chemistry by OpenStax (2015-05-04)
Additional Science Textbook Solutions
Human Anatomy & Physiology (2nd Edition)
Introductory Chemistry (6th Edition)
Chemistry: The Central Science (14th Edition)
Biology: Life on Earth (11th Edition)
Fundamentals of Anatomy & Physiology (11th Edition)
Campbell Essential Biology (7th Edition)
- can someone draw out the reaction mechanism for this reaction showing all bonds, intermediates and side products Comment on the general features of the 1H-NMR spectrum of isoamyl ester provided belowarrow_forwardWhat would be the best choices for the missing reagents 1 and 3 in this synthesis? 1. PPh3 3 2. n-BuLi • Draw the missing reagents in the drawing area below. You can draw them in any arrangement you like. • Do not draw the missing reagent 2. If you draw 1 correctly, we'll know what it is. • Note: if one of your reagents needs to contain a halogen, use bromine. Click and drag to start drawing a structure.arrow_forwardIdentify the missing organic reactants in the following reaction: X + Y H+ two steps Note: This chemical equation only focuses on the important organic molecules in the reaction. Additional inorganic or small-molecule reactants or products (like H2O) are not shown. In the drawing area below, draw the skeletal ("line") structures of the missing organic reactants X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Click and drag to start drawing a structure. Х :arrow_forward
- I am struggling with the IUPAC (sys H Reply ☑Mark as Unreadarrow_forwardDon't used hand raiting and don't used Ai solution and correct answerarrow_forwardH R Part: 1/2 :CI: is a/an electrophile Part 2 of 2 Draw the skeletal structure of the product(s) for the Lewis acid-base reaction. Include lone pairs and formal charges (if applicable) on the structures. 4-7: H ö- H Skip Part Check X :C1: $ % L Fi Click and drag to start drawing a structure. MacBook Pro & ㅁ x G 0: P Add or increase positive formal cha Save For Later Submit ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centearrow_forward
- Draw the friedel-crafts acylation mechanism of m-Xylenearrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward1. Base on this experimental results, how do you know that the product which you are turning in is methyl 3-nitrobenzoate(meta substituted product ) rather than either of the other two products? 2. What observation suggests that at least a small amount of one or both of the other two isomers are in the mother liquor?arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
