
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN: 9781938168390
Author: Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 20, Problem 42E
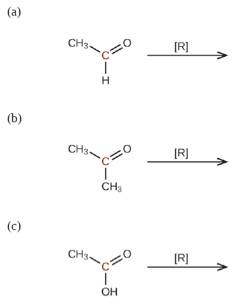
Predict the products of reducing the following molecules. In each case, identify the product that will result from the minimal decrease in oxidation state for the highlighted carbon atom:
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
x +
LEKS: Using a phase diagram a X
n/alekscgi/x/lsl.exe/10_u-IgNsikr7j8P3jH-IQs_pBan HhvlTCeeBZbufu BYTI0Hz7m7D3ZcHYUt80XL-5alyVpw
○ States of Matter
Using a phase diagram to find a phase transition temperature or pressure
Use the phase diagram of Substance X below to find the melting point of X when the pressure above the solid is 1.1 atm.
pressure (atm)
16
08-
solid
liquid-
0
200
400
gas
600
temperature (K)
Note: your answer must be within 25 °C of the exact answer to be graded correct.
×
5
S: Using a phase diagram
leksogi/x/sl.exe/1ou-IgNs kr 7j8P3jH-IQs_pBan HhvTCeeBZbufuBYTI0Hz7m7D3ZdHYU+80XL-5alyVp
O States of Matter
Using a phase diagram to find a phase transition temperature or pressure
se the phase diagram of Substance X below to find the boiling point of X when the pressure on the liquid is 1.6 atm.
pressure (atm)
32-
16-
solid
liquid
0.
gas
100
200
temperature (K)
300
Note: your answer must be within 12.5 °C of the exact answer to be graded correct.
10
Explanation
Check
§
Q Search
J
2025 McGraw Hill LLC. All Rights Rese
151.2
254.8
85.9
199.6
241.4
87.6
242.5
186.4
155.8
257.1
242.9
253.3
256.0
216.6
108.7
239.0
149.7
236.4
152.1
222.7
148.7
278.2
268.7
234.4
262.7
283.2
143.6
QUESTION: Using this group of data on salt reduced tomato sauce concentration readings answer the following questions:
1. 95% Cl Confidence Interval (mmol/L)
2. [Na+] (mg/100 mL)
3. 95% Na+ Confidence Interval (mg/100 mL)
Chapter 20 Solutions
Chemistry by OpenStax (2015-05-04)
Ch. 20 - Write the chemical formula and Lewis structure of...Ch. 20 - What is the difference between the hybridization...Ch. 20 - On a microscopic level, how does the reaction of...Ch. 20 - On a microscopic level, how does the reaction of...Ch. 20 - Explain why unbranched alkenes can form geometric...Ch. 20 - Explain why these two molecules are not isomers:Ch. 20 - Explain why these two molecules are not isomers:Ch. 20 - How does the carbon-atom hybridization change when...Ch. 20 - Write the Lewis structure and molecular formula...Ch. 20 - Write the chemical formula, condensed formula, and...
Ch. 20 - Give the complete IUPAC name for each of the...Ch. 20 - Give the complete IUPAC name for each of the...Ch. 20 - Butane is used as a fuel in disposable lighters....Ch. 20 - Write Lewis structures and name the five...Ch. 20 - Write Lewis structures for the Cis -trans isomers...Ch. 20 - Write structures for the three isomers of the...Ch. 20 - Isooctane is the common name of the isomer of...Ch. 20 - Write Lewis structures and IUPAC names for the...Ch. 20 - Write Lewis structures and IUPAC names for all...Ch. 20 - Name and write the structures of all isomers of...Ch. 20 - Write the structures for all the isomers of the...Ch. 20 - Write Lewis structures and describe the molecular...Ch. 20 - Benzene is one of the compounds used as an octane...Ch. 20 - Teflon is prepared by the polymerization of...Ch. 20 - Write two complete, balanced equations for each of...Ch. 20 - Write two complete, balanced equations for each of...Ch. 20 - What mass of 2-bromopropane could be prepared from...Ch. 20 - Acetylene is a very weak acid; however, it will...Ch. 20 - Ethylene can be produced by the pyrolysis of...Ch. 20 - Why do the compounds hexane, hexanol, and hexane...Ch. 20 - Write condensed formulas and provide IUPAC names...Ch. 20 - Give the complete IUPAC name for each of the...Ch. 20 - Give the complete IUPAC name and the common name...Ch. 20 - Write the condensed structures of both isomers...Ch. 20 - Write the condensed structures of all isomers with...Ch. 20 - Draw the condensed formulas for each of the...Ch. 20 - MTBE, Methyl tert -butyl ether, CH3OC(CH3)3, is...Ch. 20 - Write two complete balanced equations for each of...Ch. 20 - Write two complete balanced equations for each of...Ch. 20 - Order the following molecules from least to most...Ch. 20 - Predict the products of oxidizing the molecules...Ch. 20 - Predict the products of reducing the following...Ch. 20 - Explain why it is not possible to possible a...Ch. 20 - How does hybridization of the substituted carbon...Ch. 20 - Fatty acids are carboxylic acids that have long...Ch. 20 - Write a condensed structural formula, such as...Ch. 20 - Write a condensed structural formula, such as...Ch. 20 - The foul odor of rancid butter is caused by...Ch. 20 - Write the two-resonance structures for the acetate...Ch. 20 - Write two complete, balanced equations for each of...Ch. 20 - Write two complete balanced equations for each of...Ch. 20 - Yields in organic reactions are sometimes low....Ch. 20 - Alcohols A, B and C all have the composition C4H...Ch. 20 - Write the Lewis structures of both isomers with...Ch. 20 - What is the molecular structure about the nitrogen...Ch. 20 - Write the two resonance structures for the...Ch. 20 - Draw Lewis structures for pyridine and its...Ch. 20 - Write the Lewis structures of all isomers with the...Ch. 20 - Write two complete balanced equations for the...Ch. 20 - Write two complete, balanced equations for each of...Ch. 20 - Identify any carbon atoms that change...Ch. 20 - Identify any carbon atoms that change...Ch. 20 - Identify any carbon atoms that change...
Additional Science Textbook Solutions
Find more solutions based on key concepts
The central nervous system is responsible for: a. integrative functions. b. sensory functions. c. motor functio...
Human Anatomy & Physiology (2nd Edition)
Determine whether the metal in each ionic compound forms only one type of ion or more than one type of ion and ...
Introductory Chemistry (6th Edition)
Body, Heal Thyself The precision of mitotic cell division is essential for repairing damaged tissues like those...
Biology: Life on Earth with Physiology (11th Edition)
15. A good scientific hypothesis is based on existing evidence and leads to testable predictions. What hypothes...
Campbell Biology: Concepts & Connections (9th Edition)
21. Two -diameter aluminum electrodes are spaced apart.
The electrodes are connected to a battery.
...
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
5. When the phenotype of heterozygotes is intermediate between the phenotypes of the two homozygotes, this patt...
Biology: Life on Earth (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Results Search Results Best Free Coursehero Unloc xb Success Confirmation of Q x O Google Pas alekscgi/x/lsl.exe/1o_u-IgNslkr 7j8P3jH-IQs_pBanHhvlTCeeBZbufu BYTI0Hz7m7D3ZcHYUt80XL-5alyVpwDXM TEZayFYCavJ17dZtpxbFD0Qggd1J O States of Matter Using a phase diagram to find a phase transition temperature or pressure Gabr 3/5 he pressure above a pure sample of solid Substance X at 101. °C is lowered. At what pressure will the sample sublime? Use the phase diagram of X below to nd your answer. pressure (atm) 24- 12 solid liquid gas 200 400 temperature (K) 600 ote: your answer must be within 0.15 atm of the exact answer to be graded correct. atm Thanation Check © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center I Q Search L³ ملةarrow_forward301.7 348.9 193.7 308.6 339.5 160.6 337.7 464.7 223.5 370.5 326.6 327.5 336.1 317.9 203.8 329.8 221.9 331.7 211.7 309.6 223.4 353.7 334.6 305.6 340.0 304.3 244.7 QUESTION: Using this group of data on regular tomato sauce concentration readings answer the following questions: 1. 95% Cl Confidence Interval (mmol/L) 2. [Na+] (mg/100 mL) 3. 95% Na+ Confidence Interval (mg/100 mL)arrow_forwardSearch Results Search Results Best Free Coursehero Unlo x b Success Confirmation of Q aleks.com/alekscgi/x/sl.exe/10_u-lgNslkr7j8P3jH-IQs_pBan HhvlTCeeBZbufu BYTIOHz7m7D3ZcHYUt80XL-5alyVpwDXM TEZayFYCav States of Matter Using a phase diagram to find a phase transition temperature or pressure Use the phase diagram of Substance X below to find the temperature at which X turns to a gas, if the pressure above the solid is 3.7 atm. pressure (atm) 0. 32- 16 solid liquid gas 200 temperature (K) Note: your answer must be within 20 °C of the exact answer to be graded correct. Дос Xarrow_forward
- Consider the reaction below to answer the following questions: Acetoacetic ester can be prepared by the Claisen self-condensation reaction of ethyl acetate. 1. NaOEt, EtOH H&C OCH CH3 2 H30 H3C CH2 OCH2CH3 A. Write the complete stepwise mechanism for this reaction. Show all electron flow with arrows and draw all intermediate structures. B. Ethyl acetate can be prepared from ethanol as the only organic starting material. Show all reagents and structures for all intermediates in this preparation. C. Give the structures of the ester precursors for the following Claisen condensation product and formulate the reaction. OEtarrow_forwardUse the phase diagram of Substance X below to find the temperature at which X turns to a gas, if the pressure above the solid is 3.7 atm. pressure (atm) 32 16 solid liquid gas 0 0 200 temperature (K) Note: your answer must be within 20 °C of the exact answer to be graded correct. Шос ☑ كarrow_forwardStarting from bromoethane, how could you prepare the following compounds: a. Ethanol. b. Acetaldehyde f. Acetone. e. 2-Propanol i. Acetoacetic ester. d. 2-Bromoacetic acid. c. Acetic acid g. Acetamide. j. Ethylmalonate k. Gama ketoacid. h. Ethyl magnesium bromide.arrow_forward
- - The pressure above a pure sample of solid Substance X at 60. °C is raised. At what pressure will the sample melt? Use the phase diagram of X below to find your answer. pressure (atm) 02 0.4 solid Hliquid gas 0 0 200 400 600 temperature (K) Note: your answer must be within 0.025 atm of the exact answer to be graded correct. ☐ atmarrow_forward15. What is the order of decreasing reactivity towards nucleophilic acyl substitution for the carboxylic acid derivatives? (most reactive first) 0 O H3C COC CH3 H₂C C N(CH3)2 H3C C OCH3 A. a. I, 11, 111, b. I, III, IV, II C. II, IV, III, I ° (CH3)2CH C OCH3 IV d. II, I, III, IV B. R COCR 0 0 0 13= RC NH2 RC OR RC CI === IV a. I, III, II, IV b. II, III, I, IV C. III, II, I, IV d. IV, I, III, IIarrow_forwardDraw the formula of the product obtained by reacting D-Tallose with bromine water.arrow_forward
- Choose the best reagent(s) for carrying out the following conversions from the list below. Place the letter corresponding to the best choice in the blank to the left of the conversion. a. KMnO4, H3O+ b. Tollens' Reagent [oxidizing reagent] C. NaBH4, ethanol d. 1. BH3 2. H3O+ e. 1. CH3MgBr, ether 2. H3O+ f. CrO3, H2SO4, H₂O g. 1. Mg, ether 2. CO2 3. H3O+ h. 1. NaCN 2. H2SO4, H2, heat i. O3, then Zn and HOAC j. CH₂I A. B. C. CH CH=CHCH2COOH Br CEN CH COOH + HOOCCH COOH COOH 010 CH3arrow_forwardDraw the structures for each of the intermediates in the boxes provided for the synthesis below. OCH3 Fe HO HNO (CHOO pynding H₂504 LHNO2 NACH-I Fa H₂O HCL HNO 180arrow_forwardProvide structure(s) for the starting material(s), reagent(s) or the major organic product(s) of each of the following reactions or sequences of reactions. Show all relevant stereochemistry [three only] A. o 11 (CH3)CH — C— C ether (CH3)2CH-C-O-C-CH3 B. CH3 CHy CI Staf OH C. HC OCHS + H₂Oarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License