
Concept explainers
What is meant by the term “unsaturated hydrocarbon”? What structural feature characterizes
Interpretation:
The meaning of “unsaturated hydrocarbon” should be determined along with the structural features which characterizes unsaturated hydrocarbons.
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Hydrocarbons are classified as saturated hydrocarbon and unsaturated hydrocarbon
Answer to Problem 1ALQ
The hydrocarbons in which carbon-carbon multiple bonds are present is said to be unsaturated hydrocarbons.
The unsaturated hydrocarbon which contains one or more double bonds in the structure is known as alkene whereas which contains one or more triple bonds in the structure is known as alkyne.
Explanation of Solution
Unsaturated hydrocarbons are those hydrocarbons in which carbon-carbon multiple bonds are present that is double and triple bond.
Unsaturated hydrocarbon which contains one or more double bond is known as alkenes and which contains one or more triple bonds are said to be alkynes.
Carbon atoms linked with double bond are bound with each other by one sigma and one pi bond and carbon atoms linked with triple bond are bound with each other by one sigma and two pi bonds.
For example:
The structure of alkene and alkyne is given as:
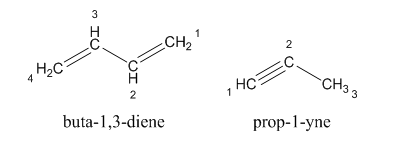
The hydrocarbons in which carbon-carbon multiple bonds are present is said to be unsaturated hydrocarbons.
The unsaturated hydrocarbon which contains one or more double bonds in the structure is known as alkene whereas which contains one or more triple bonds in the structure is known as alkyne.
Want to see more full solutions like this?
Chapter 20 Solutions
Introductory Chemistry: Foundation - Text (Looseleaf)
Additional Science Textbook Solutions
Microbiology: Principles and Explorations
College Physics: A Strategic Approach (3rd Edition)
Brock Biology of Microorganisms (15th Edition)
SEELEY'S ANATOMY+PHYSIOLOGY
Organic Chemistry (8th Edition)
- Redraw the molecule below as a skeletal ("line") structure. Be sure to use wedge and dash bonds if necessary to accurately represent the direction of the bonds to ring substituents. Cl. Br Click and drag to start drawing a structure. : ☐ ☑ Parrow_forwardK m Choose the best reagents to complete the following reaction. L ZI 0 Problem 4 of 11 A 1. NaOH 2. CH3CH2CH2NH2 1. HCI B OH 2. CH3CH2CH2NH2 DII F1 F2 F3 F4 F5 A F6 C CH3CH2CH2NH2 1. SOCl2 D 2. CH3CH2CH2NH2 1. CH3CH2CH2NH2 E 2. SOCl2 Done PrtScn Home End FA FQ 510 * PgUp M Submit PgDn F11arrow_forwardNonearrow_forward
- Add curved arrows to the reactants in this reaction. A double-barbed curved arrow is used to represent the movement of a pair of electrons. Draw curved arrows. : 0: si H : OH :: H―0: Harrow_forwardConsider this step in a radical reaction: Br N O hv What type of step is this? Check all that apply. Draw the products of the step on the right-hand side of the drawing area below. If more than one set of products is possible, draw any set. Also, draw the mechanism arrows on the left-hand side of the drawing area to show how this happens. O primary Otermination O initialization O electrophilic O none of the above × ☑arrow_forwardNonearrow_forward
- Can I get a drawing of what is happening with the orbitals (particularly the p orbital) on the O in the OH group? Is the p orbital on the O involved in the ring resonance? Why or why not?arrow_forward1) How many monochlorination products-including stereochemistry- are there for the molecule below:arrow_forwardSelect an amino acid that has and N-H or O-H bond in its R-group (you have 8 to choose from!). Draw at least two water molecules interacting with the R-group of the amino acid.arrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning





