
Concept explainers
(a)
Interpretation:
Name of the given organic compound should be determined.
Concept Introduction:
The group that contains carboxyl group which is attached to at least one hydrogen is said to be an
In order to give the name to the aldehyde group, the following steps are followed:
1. The parent (longest)
2. The ending of the parent chain from alkane (-e) is changed to -al for an aldehyde group. The carbonyl group of an aldehyde appear at the end of the carbon chain so, the numbering start with carbon having aldehyde group.
3. Name should be written in alphabetical order and other substituents are shown by the number.
For number of carbons atoms chain, the prefix is given as:
Carbon-1meth
Carbon-2eth
Carbon-3prop
Carbon-4but
Carbon-5pent
Carbon-6hex
Carbon-7hept
Carbon-8oct
Carbon-9non
Carbon-10dec.
Answer to Problem 145CP
Name of the given compound is:
Heptanal.
Explanation of Solution
The given structure is:
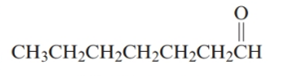
The parent chain in the given structure is heptane. Numbering is done in such a way that carbonyl carbon gets number 1.
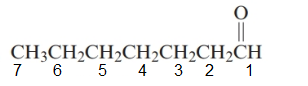
So, the name will be: heptanal.
(b)
Interpretation:
Name of the given organic compound should be determined.
Concept Introduction:
The group that contains carboxyl group which is attached to at least one hydrogen is said to be an aldehyde group, general representation of an aldehyde group is RCH=O or RCHO. Whereas the group that contains carboxyl group which is attached to two carbon atoms is said to be a ketone group, general representation of a ketone group is RCOR’.
In order to give the name to the aldehyde group, the following steps are followed:
1. The parent (longest) alkane chain is identified.
2. The ending of the parent chain from alkane (-e) is changed to -al for an aldehyde group. The carbonyl group of an aldehyde appear at the end of the carbon chain so, the numbering start with carbon having aldehyde group.
3. Name should be written in alphabetical order and other substituents are shown by the number.
For number of carbons atoms chain, the prefix is given as:
Carbon-1meth
Carbon-2eth
Carbon-3prop
Carbon-4but
Carbon-5pent
Carbon-6hex
Carbon-7hept
Carbon-8oct
Carbon-9non
Carbon-10dec.
Answer to Problem 145CP
Name of the given compound is:
3-ethyl-5-methylhexanal.
Explanation of Solution
The given structure is:
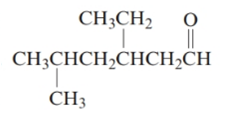
The parent chain in the given structure is hexane. Numbering is done in such a way that carbonyl carbon gets number 1.
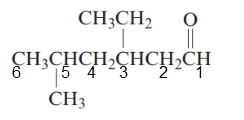
The substituent on 3 position is ethyl and on 5 position is methyl.
So, the name will be: 3-ethyl-5-methylhexanal.
(c)
Interpretation:
Name of the given organic compound should be determined.
Concept Introduction:
The group that contains carboxyl group which is attached to at least one hydrogen is said to be an aldehyde group, general representation of an aldehyde group is RCH=O or RCHO. Whereas the group that contains carboxyl group which is attached to two carbon atoms is said to be a ketone group, general representation of a ketone group is RCOR’.
In order to give the name to the ketone group, the following steps are followed:
1. The parent (longest) alkane chain is identified.
2. The ending of the parent chain from alkane (-e) is changed to -one for a ketone group.
3. The numbering is of the chain is done in such a way that carbonyl carbon gets the smaller number.
4. Name should be written in alphabetical order and other substituents are shown by the number.
For number of carbons atoms chain, the prefix is given as:
Carbon-1meth
Carbon-2eth
Carbon-3prop
Carbon-4but
Carbon-5pent
Carbon-6hex
Carbon-7hept
Carbon-8oct
Carbon-9non
Carbon-10dec.
Answer to Problem 145CP
Name of the given compound is:
Hexan-3-one.
Explanation of Solution
The given structure is:
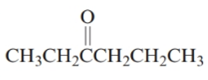
The parent chain in the given structure is hexane. Numbering is done in such a way that carbonyl carbon gets lower number that is 3.
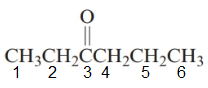
So, the name will be: Hexan-3-one.
(d)
Interpretation:
Name of the given organic compound should be determined.
Concept Introduction:
The group that contains carboxyl group which is attached to at least one hydrogen is said to be an aldehyde group, general representation of an aldehyde group is RCH=O or RCHO. Whereas the group that contains carboxyl group which is attached to two carbon atoms is said to be a ketone group, general representation of a ketone group is RCOR’.
In order to give the name to the ketone group, the following steps are followed:
1. The parent (longest) alkane chain is identified.
2. The ending of the parent chain from alkane (-e) is changed to -one for a ketone group.
3. The numbering is of the chain is done in such a way that carbonyl carbon gets the smaller number.
4. Name should be written in alphabetical order and other substituents are shown by the number.
For number of carbons atoms chain, the prefix is given as:
Carbon-1meth
Carbon-2eth
Carbon-3prop
Carbon-4but
Carbon-5pent
Carbon-6hex
Carbon-7hept
Carbon-8oct
Carbon-9non
Carbon-10dec.
Answer to Problem 145CP
Name of the given compound is:
4, 5-dimethylhexan-3-one.
Explanation of Solution
The given structure is:
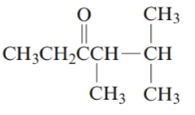
The parent chain in the given structure is hexane. Numbering is done in such a way that carbonyl carbon gets lower number that is 3.
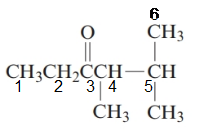
The substituent on 4 and 5 position is methyl.
So, the name will be: 4, 5-dimethylhexan-3-one.
(e)
Interpretation:
Name of the given organic compound should be determined.
Concept Introduction:
An organic compound in which carboxy
In order to give the name to the carboxylic acid group, the following steps are followed:
1. The parent (longest) alkane chain is identified.
2. The ending of the parent chain from alkane (-e) is changed to -oic acid for a carboxylic acid group.
3. The numbering is of the chain is done in such a way that carbonyl carbon gets the smaller number.
4. Name should be written in alphabetical order and other substituents are shown by the number.
For number of carbons atoms chain, the prefix is given as:
Carbon-1meth
Carbon-2eth
Carbon-3prop
Carbon-4but
Carbon-5pent
Carbon-6hex
Carbon-7hept
Carbon-8oct
Carbon-9non
Carbon-10dec.
Answer to Problem 145CP
Name of the given compound is:
3, 4-dimethylhexanoic acid.
Explanation of Solution
The given structure is:
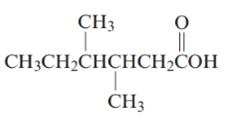
The parent chain in the given structure is hexane. Numbering is done in such a way that carboxyl group gets number 1.
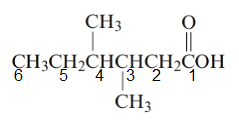
The substituent on 3 and 4 position is methyl.
So, the name will be: 3, 4-dimethylhexanoic acid.
Want to see more full solutions like this?
Chapter 20 Solutions
Introductory Chemistry: Foundation - Text (Looseleaf)
- 4. Provide a clear arrow-pushing mechanism for each of the following reactions. Do not skip proton transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted without ambiguity. a. 2. 1. LDA 3. H3O+ HOarrow_forwardb. H3C CH3 H3O+ ✓ H OHarrow_forward2. Provide reagents/conditions to accomplish the following syntheses. More than one step is required in some cases. a. CH3arrow_forward
- Identify and provide an explanation that distinguishes a qualitative and quantitative chemical analysis. Provide examples.arrow_forwardIdentify and provide an explanation of the operational principles behind a Atomic Absorption Spectrometer (AAS). List the steps involved.arrow_forwardInstructions: Complete the questions in the space provided. Show all your work 1. You are trying to determine the rate law expression for a reaction that you are completing at 25°C. You measure the initial reaction rate and the starting concentrations of the reactions for 4 trials. BrO³¯ (aq) + 5Br¯ (aq) + 6H* (aq) → 3Br₂ (l) + 3H2O (l) Initial rate Trial [BrO3] [H*] [Br] (mol/L) (mol/L) | (mol/L) (mol/L.s) 1 0.10 0.10 0.10 8.0 2 0.20 0.10 0.10 16 3 0.10 0.20 0.10 16 4 0.10 0.10 0.20 32 a. Based on the above data what is the rate law expression? b. Solve for the value of k (make sure to include proper units) 2. The proposed reaction mechanism is as follows: i. ii. BrО¸¯ (aq) + H+ (aq) → HBrO3 (aq) HBrO³ (aq) + H* (aq) → H₂BrO3* (aq) iii. H₂BrO³* (aq) + Br¯ (aq) → Br₂O₂ (aq) + H2O (l) [Fast] [Medium] [Slow] iv. Br₂O₂ (aq) + 4H*(aq) + 4Br(aq) → 3Br₂ (l) + H2O (l) [Fast] Evaluate the validity of this proposed reaction. Justify your answer.arrow_forward
- a. H3C CH3 H, 1.0 equiv. Br2arrow_forwardH3C. H3C CH 3 CH 3 CH3 1. LDA 2. PhSeCl 3. H2O2arrow_forwardPlease predict the products for each of the following reactions: 1.03 2. H₂O NaNH, 1. n-BuLi 2. Mel A H₂ 10 9 0 H2SO4, H₂O HgSO4 Pd or Pt (catalyst) B 9 2 n-BuLi ♡ D2 (deuterium) Lindlar's Catalyst 1. NaNH2 2. EtBr Na, ND3 (deuterium) 2. H₂O2, NaOH 1. (Sia)2BH с Darrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning  Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





