
Concept explainers
(a)
Interpretation:
The systematic names of the given
Concept Introduction:
The hydrocarbon compounds that is compound containing only hydrogen and carbon atoms that contains multiple bond(s) are said to be unsaturated hydrocarbon. Compounds containing double bonds are said to be
In order to give the name to the unsaturated hydrocarbon following steps are followed:
1. The parent (longest) continuous carbon chain containing multiple bonds between the carbon atoms is selected.
2. While writing the name of alkene, the suffix “ane” of the corresponding
3. Name should be written in alphabetical order and numbering should be done in such a way that the multiple bond and substituent group gets lowest number.
4. Hyphen is used to connect the number to the name.
For number of carbons atoms in alkane chain, the prefix is given as:
Carbon-1meth
Carbon-2eth
Carbon-3prop
Carbon-4but
Carbon-5pent
Carbon-6hex
Carbon-7hept
Carbon-8oct
Carbon-9non
Carbon-10dec.
Answer to Problem 144CP
2-methylbut-1-ene.
Explanation of Solution
The given structure is:
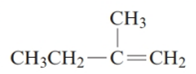
The parent chain in the given structure is of 4-carbon atoms so, prefix will be but. Numbering is done in such a way that the multiple bond that is double bond gets lower number that is 1 for double bond and the substituent on 2 position is methyl.
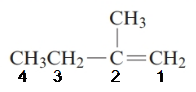
So, the IUPAC name will be: 2-methylbut-1-ene.
(b)
Interpretation:
The systematic names of the given unsaturated hydrocarbon should be determined.
Concept Introduction:
The hydrocarbon compounds that is compound containing only hydrogen and carbon atoms that contains multiple bond(s) are said to be unsaturated hydrocarbon. Compounds containing double bonds are said to be alkene whereas compounds containing triple bonds are said to be alkyne.
In order to give the name to the unsaturated hydrocarbon following steps are followed:
1. The parent (longest) continuous carbon chain containing multiple bonds between the carbon atoms is selected.
2. While writing the name of alkene, the suffix “ane” of the corresponding alkane is replaced by “ene”.
3. Name should be written in alphabetical order and numbering should be done in such a way that the multiple bond and substituent group gets lowest number.
4. Hyphen is used to connect the number to the name.
For number of carbons atoms in alkane chain, the prefix is given as:
Carbon-1meth
Carbon-2eth
Carbon-3prop
Carbon-4but
Carbon-5pent
Carbon-6hex
Carbon-7hept
Carbon-8oct
Carbon-9non
Carbon-10dec.
Answer to Problem 144CP
2, 4-dimethylpent-1, 4-diene.
Explanation of Solution
The given structure is:
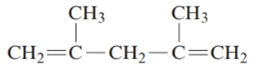
The parent chain in the given structure is of 5-carbon atoms so, prefix will be pent. Numbering is done in such a way that the multiple bond that is double bond gets lower number that is 1 and 4 for double bond and the substituent on 2 and 4 position is methyl.
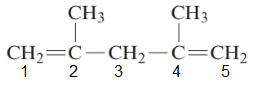
So, the IUPAC name will be: 2, 4-dimethylpent-1, 4-diene.
(c)
Interpretation:
The systematic names of the given unsaturated hydrocarbon should be determined.
Concept Introduction:
The hydrocarbon compounds that is compound containing only hydrogen and carbon atoms that contains multiple bond(s) are said to be unsaturated hydrocarbon. Compounds containing double bonds are said to be alkene whereas compounds containing triple bonds are said to be alkyne.
In order to give the name to the unsaturated hydrocarbon following steps are followed:
1. The parent (longest) continuous carbon chain containing multiple bonds between the carbon atoms is selected.
2. While writing the name of alkene, the suffix “ane” of the corresponding alkane is replaced by “ene”.
3. Name should be written in alphabetical order and numbering should be done in such a way that the multiple bond and substituent group gets lowest number.
4. Hyphen is used to connect the number to the name.
For number of carbons atoms in alkane chain, the prefix is given as:
Carbon-1meth
Carbon-2eth
Carbon-3prop
Carbon-4but
Carbon-5pent
Carbon-6hex
Carbon-7hept
Carbon-8oct
Carbon-9non
Carbon-10dec.
Answer to Problem 144CP
6-ethyl-2-methyloct-4-ene.
Explanation of Solution
The given structure is:
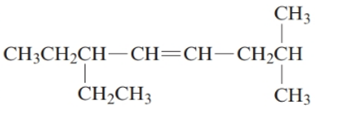
The parent chain in the given structure is of 5-carbon atoms so, prefix will be oct. Numbering is done in such a way that the multiple bond that is double bond gets lower number that is 4 for double bond and the substituent on 2 position is methyl and on 6 position is ethyl.
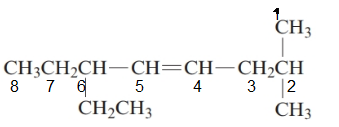
So, the IUPAC name will be: 6-ethyl-2-methyloct-4-ene.
(d)
Interpretation:
The systematic names of the given unsaturated hydrocarbon should be determined.
Concept Introduction:
The hydrocarbon compounds that is compound containing only hydrogen and carbon atoms that contains multiple bond(s) are said to be unsaturated hydrocarbon. Compounds containing double bonds are said to be alkene whereas compounds containing triple bonds are said to be alkyne.
In order to give the name to the unsaturated hydrocarbon following steps are followed:
1. The parent (longest) continuous carbon chain containing multiple bonds between the carbon atoms is selected.
2. While writing the name of alkyne, the suffix “ane” of the corresponding alkane is replaced by “yne”.
3. Name should be written in alphabetical order and numbering should be done in such a way that the multiple bond and substituent group gets lowest number.
4. Hyphen is used to connect the number to the name.
For number of carbons atoms in alkane chain, the prefix is given as:
Carbon-1meth
Carbon-2eth
Carbon-3prop
Carbon-4but
Carbon-5pent
Carbon-6hex
Carbon-7hept
Carbon-8oct
Carbon-9non
Carbon-10dec.
Answer to Problem 144CP
3-bromohept-1-yne.
Explanation of Solution
The given structure is:
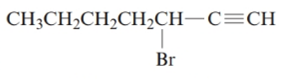
The parent chain in the given structure is of 7-carbon atoms so, prefix will be hept. Numbering is done in such a way that the multiple bond that is triple bond gets lower number that is 1 for triple bond. The substituent on 3 position is bromo.
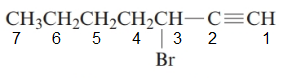
So, the IUPAC name will be: 3-bromohept-1-yne.
(e)
Interpretation:
The systematic names of the given unsaturated hydrocarbon should be determined.
Concept Introduction:
The hydrocarbon compounds that is compound containing only hydrogen and carbon atoms that contains multiple bond(s) are said to be unsaturated hydrocarbon. Compounds containing double bonds are said to be alkene whereas compounds containing triple bonds are said to be alkyne.
In order to give the name to the unsaturated hydrocarbon following steps are followed:
1. The parent (longest) continuous carbon chain containing multiple bonds between the carbon atoms is selected.
2. While writing the name of alkyne, the suffix “ane” of the corresponding alkane is replaced by “yne”.
3. Name should be written in alphabetical order and numbering should be done in such a way that the multiple bond and substituent group gets lowest number.
4. Hyphen is used to connect the number to the name.
For number of carbons atoms in alkane chain, the prefix is given as:
Carbon-1meth
Carbon-2eth
Carbon-3prop
Carbon-4but
Carbon-5pent
Carbon-6hex
Carbon-7hept
Carbon-8oct
Carbon-9non
Carbon-10dec.
Answer to Problem 144CP
7-chloro-2, 5, 5-trimethylhept-3-yne.
Explanation of Solution
The given structure is:
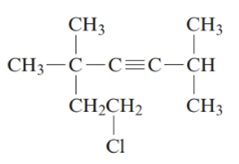
The parent chain in the given structure is of 7-carbon atoms so, prefix will be hept. Numbering is done in such a way that the multiple bond that is triple bond gets lower number that is 3 for triple bond. The substituent on 2 and 5 position is methyl and on 7 position is chloro.
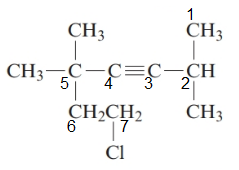
So, the IUPAC name will be: 7-chloro-2, 5, 5-trimethylhept-3-yne.
(f)
Interpretation:
The systematic names of the given unsaturated hydrocarbon should be determined.
Concept Introduction:
The hydrocarbon compounds that is compound containing only hydrogen and carbon atoms that contains multiple bond(s) are said to be unsaturated hydrocarbon. Compounds containing double bonds are said to be alkene whereas compounds containing triple bonds are said to be alkyne.
In order to give the name to the unsaturated hydrocarbon following steps are followed:
1. The parent (longest) continuous carbon chain containing multiple bonds between the carbon atoms is selected.
2. While writing the name of alkyne, the suffix “ane” of the corresponding alkane is replaced by “yne”.
3. Name should be written in alphabetical order and numbering should be done in such a way that the multiple bond and substituent group gets lowest number.
4. Hyphen is used to connect the number to the name.
For number of carbons atoms in alkane chain, the prefix is given as:
Carbon-1meth
Carbon-2eth
Carbon-3prop
Carbon-4but
Carbon-5pent
Carbon-6hex
Carbon-7hept
Carbon-8oct
Carbon-9non
Carbon-10dec.
Answer to Problem 144CP
4-ethyl-3-methyloct-1-yne.
Explanation of Solution
The given structure is:
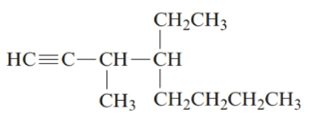
The parent chain in the given structure is of 8-carbon atoms so, prefix will be oct. Numbering is done in such a way that the multiple bond that is triple bond gets lower number that is 1 for triple bond. The substituent on 3 position is methyl and on 4 position is ethyl.
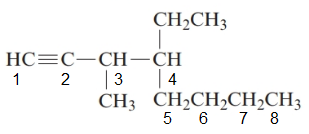
So, the IUPAC name will be: 4-ethyl-3-methyloct-1-yne.
Want to see more full solutions like this?
Chapter 20 Solutions
Introductory Chemistry: Foundation - Text (Looseleaf)
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





