
Concept explainers
Interpretation:
Using the molecular modeling kit, the given molecule is to be constructed, and then after each successive
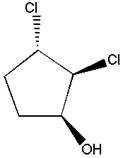
Concept introduction:
A straight line represents a bond that is in the plane of the paper. A wedge signifies a bond, which points toward you and comes out of the plane of the paper. A dash signifies a bond which points away from you and goes behind the plane of the paper. A
Want to see the full answer?
Check out a sample textbook solution
Chapter 2 Solutions
Organic Chemistry: Principles And Mechanisms: Study Guide/solutions Manual (second)
- CS2(g) → CS(g) + S(g) The rate law is Rate = k [CS2] where k = 1.6 × 10-6 s−1. S Calculate the half-life.arrow_forwardThe following is a first order reaction where the rate constant, k, is 6.29 x 10-3 min-*** What is the half-life? C2H4 C2H2 + H2arrow_forwardControl Chart Drawing Assignment The table below provides the number of alignment errors observed during the final inspection of a certain model of airplane. Calculate the central, upper, and lower control limits for the c-chart and draw the chart precisely on the graph sheet provided (based on 3-sigma limits). Your chart should include a line for each of the control limits (UCL, CL, and LCL) and the points for each observation. Number the x-axis 1 through 25 and evenly space the numbering for the y-axis. Connect the points by drawing a line as well. Label each line drawn. Airplane Number Number of alignment errors 201 7 202 6 203 6 204 7 205 4 206 7 207 8 208 12 209 9 210 9 211 8 212 5 213 5 214 9 215 8 216 15 217 6 218 4 219 13 220 7 221 8 222 15 223 6 224 6 225 10arrow_forward
- Collagen is used to date artifacts. It has a rate constant = 1.20 x 10-4 /years. What is the half life of collagen?arrow_forwardיווי 10 20 30 40 50 60 70 3.5 3 2.5 2 1.5 1 [ppm] 3.5 3 2.5 2 1.5 1 6 [ppm] 1 1.5 -2.5 3.5arrow_forward2H2S(g)+3O2(g)→2SO2(g)+2H2O(g) A 1.2mol sample of H2S(g) is combined with excess O2(g), and the reaction goes to completion. Question Which of the following predicts the theoretical yield of SO2(g) from the reaction? Responses 1.2 g Answer A: 1.2 grams A 41 g Answer B: 41 grams B 77 g Answer C: 77 grams C 154 g Answer D: 154 grams Darrow_forward
- Part VII. Below are the 'HNMR, 13 C-NMR, COSY 2D- NMR, and HSQC 2D-NMR (similar with HETCOR but axes are reversed) spectra of an organic compound with molecular formula C6H1003 - Assign chemical shift values to the H and c atoms of the compound. Find the structure. Show complete solutions. Predicted 1H NMR Spectrum 4.7 4.6 4.5 4.4 4.3 4.2 4.1 4.0 3.9 3.8 3.7 3.6 3.5 3.4 3.3 3.2 3.1 3.0 2.9 2.8 2.7 2.6 2.5 2.4 2.3 2.2 2.1 2.0 1.9 1.8 1.7 1.6 1.5 1.4 1.3 1.2 1.1 f1 (ppm) Predicted 13C NMR Spectrum 100 f1 (ppm) 30 220 210 200 190 180 170 160 150 140 130 120 110 90 80 70 -26 60 50 40 46 30 20 115 10 1.0 0.9 0.8 0 -10arrow_forwardQ: Arrange BCC and Fec metals, in sequence from the Fable (Dr. R's slides) and Calculate Volume and Density. Aa BCC V 52 5 SFCCarrow_forwardNonearrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
