
Concept explainers
Propose structures for molecules that meet the following descriptions. Assume that the kinds of carbons (1°, 2°, 3°, or 4°) have been assigned by DEPT-NMR.
a) C6H12O;IR: 1715 cm-1; 13C NMR: 8.0 δ (1°), 18.5 δ (1°), 33.5 δ (2°), 40.6 δ (3°), 214.0 δ (4°)
b) C5H10O; IR: 1730 cm-1; 13C NMR: 22.6δ (1°), 23.6 8 (3°), 52.8 8 (2°), 202.4 8 (3°)
c) C6H8O; IR: 1680 cm-1; 13C NMR: 22.9 δ (2°), 25.8 8 (2°), 38.2 δ (2°). 129.8 8 (3°), 150.6 8 (3°), 198.7 δ (4°)
a)

Interpretation:
A structure for the molecule which meets the descriptions given is to be assigned.
C6H12O; IR: 1715 cm-1; 13CNMR: 8.0 δ (10), 18.5 δ (10), 33.5 δ (20), 40.6 δ (30), 214.0 δ (40)
Concept introduction:
Aldehydes and ketones show a strong absorption band in IR from 1660-1770 cm-1. Aldehydes show two characteristic C-H absorptions between 2700-2760cm-1 and 2800-2860 cm-1. Saturated aldehydes absorb near 1730 cm-1 while aromatic aldehydes and α, β- unsaturated aldehydes absorb near 1705 cm-1. Saturated ketones and cyclohexanones absorb near 1715 cm-1 while aromatic ketones and α, β- unsaturated ketones absorb near 1685-1690 cm-1. Cyclopentanones absorb around 1750 cm-1.
In 13CNMR, carbons in double bond which are sp2 hybridized absorb from 110 to 220 δ. Saturated aldehydes and ketones usually absorb in the region from 200 to 215 δ While aromatic and unsaturated carbonyl compounds absorb in the 190 to 200 δ region. The primary alkyl carbon absorbs in the range 10-15 δ, a secondary alkyl radical in the range 16-25 δ while a tertiary alkyl in the range 25-35 δ. The signals will be observed at downfield if these alkyl groups are attached to electron withdrawing groups.
To assign:
A structure for the molecule which meets the descriptions given.
C6H12O; IR: 1715 cm-1; 13CNMR: 8.0 δ (10), 18.5 δ (10), 33.5 δ (20), 40.6 δ (30), 214.0 δ (40)
Answer:
Answer to Problem 69AP
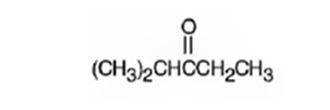
A structure for the molecule which meets the spectral descriptions given is
Explanation of Solution
The molecular formula of the compound is C6H12O. Ignoring oxygen the molecular formula of the parent compound becomes C6H14. Thus the compound has two hydrogen atoms (14-12) less than the parent and hence has one unsaturation unit perhaps a C=O.
The IR absorption at 1715 cm-1 and 13CNMR peak at 214.0 δ indicate that the compound is a ketone. The 13CNMR further indicates that the carbonyl group is flanked by a secondary and tertiary carbon indicated by the two downfield signals at 33.5 δ and 40.6 δ. The remaining three carbons has to be primary, of which one is attached as methyl(8.0 δ) to the secondary carbon and two others are attached as two methyl groups (18.5δ) on the tertiary carbon. Hence the compound is 2-methyl-3-pentanone.
A structure for the molecule which meets the spectral descriptions given is

b)

Interpretation:
A structure for the molecule which meets the descriptions given is to be assigned.
C5H10O; IR: 1730 cm-1; 13CNMR: 22.6 δ (10), 23.6 δ (30), 52.8 δ (20), 202.4 δ (30).
Concept introduction:
Aldehydes and ketones show a strong absorption band in IR from 1660-1770 cm-1. Aldehydes show two characteristic C-H absorptions between 2700-2760cm-1 and 2800-2860 cm-1. Saturated aldehydes absorb near 1730 cm-1 while aromatic aldehydes and α, β- unsaturated aldehydes absorb near 1705 cm-1. Saturated ketones and cyclohexanones absorb near 1715 cm-1 while aromatic ketones and α, β- unsaturated ketones absorb near 1685-1690 cm-1. Cyclopentanones absorb around 1750 cm-1.
In 13CNMR, carbons in double bond which are sp2 hybridized absorb from 110 to 220 δ. Saturated aldehydes and ketones usually absorb in the region from 200 to 215 δ While aromatic and unsaturated carbonyl compounds absorb in the 190 to 200 δ region. The primary alkyl carbon absorbs in the range 10-15 δ, a secondary alkyl radical in the range 16-25 δ while a tertiary alkyl in the range 25-35 δ. The signals will be observed at downfield if these alkyl groups are attached to electron withdrawing groups.
To assign:
A structure for the molecule which meets the descriptions given.
C5H10O; IR: 1730 cm-1; 13CNMR: 22.6 δ (10), 23.6 δ (30), 52.8 δ (20), 202.4 δ (30).
Answer to Problem 69AP
A structure for the molecule which meets the spectral descriptions given is

Explanation of Solution
The molecular formula of the compound is C6H12O. Ignoring oxygen the molecular formula of the parent compound becomes C6H14. Thus the compound has two hydrogen atoms less than the parent and hence has one unsaturation unit, a carbonyl group.
The IR absorption at 1730 cm-1 and 13CNMR peak at 202.4 δ indicate that the compound is a saturated aldehyde. The 13CNMR further indicates that the carbonyl group is attached to a secondary carbon indicated by the downfield signal at 52.8 δ. Of the remaining three carbons one has to be tertiary(23.6 δ) which is attached to two methyl groups(22.6 δ). Hence the compound is 2-methylbutanal.
A structure for the molecule which meets the spectral descriptions given is

c)
Interpretation:
A structure for the molecule which meets the descriptions given is to be assigned.
C6H8O; IR: 1680 cm-1; 13CNMR: 22.9 δ (20), 25.8 δ (20), 38.2 δ (20), 129.8 δ (30) 150.6 δ (30), 198.7 δ (40).
Concept introduction:
Aldehydes and ketones show a strong absorption band in IR from 1660-1770 cm-1. Aldehydes show two characteristic C-H absorptions between 2700-2760cm-1 and 2800-2860 cm-1. Saturated aldehydes absorb near 1730 cm-1 while aromatic aldehydes and α, β- unsaturated aldehydes absorb near 1705 cm-1. Saturated ketones and cyclohexanones absorb near 1715 cm-1 while aromatic ketones and α, β- unsaturated ketones absorb near 1685-1690 cm-1. Cyclopentanones absorb around 1750 cm-1.
In 13CNMR, carbons in double bond which are sp2 hybridized absorb from 110 to 220 δ. Saturated aldehydes and ketones usually absorb in the region from 200 to 215 δ While aromatic and unsaturated carbonyl compounds absorb in the 190 to 200 δ region. The primary alkyl carbon absorbs in the range 10-15 δ, a secondary alkyl radical in the range 16-25 δ while a tertiary alkyl in the range 25-35 δ.
To assign:
A structure for the molecule which meets the descriptions given.
C6H8O; IR: 1680 cm-1; 13CNMR: 22.9 δ (20), 25.8 δ (20), 38.2 δ (20), 129.8 δ (30) 150.6 δ (30), 198.7 δ (40).
Answer to Problem 69AP
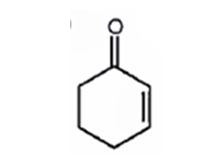
A structure for the molecule which meets the spectral descriptions given is
Explanation of Solution
The molecular formula of the compound is C6H8O. Ignoring oxygen the molecular formula of the parent compound becomes C6H14. Thus the compound has six hydrogen atoms (14-8= 6) less than the parent and hence has three unsaturation units.
The IR absorption at 1680 cm-1 and 13CNMR peak at 198.7 δ indicate that the compound is an α, β- unsaturatedketone. The compound has three unsaturated units. α, β- unsaturatedketone accounts for two unsaturation units. Hence the compound contains a six membered ring. The signals at 129.8 δ (30) 150.6 δ (30) can be attributed to the carbons in double bond. The other three signals at 22.9 δ (20), 25.8 δ (20), 38.2 δ (20) can be attributed to three methylene groups. The signal at 38.2 δ (20) can be assigned to methylene carbon attached to carbonyl carbon, that at 25.8 δ (20) to the methylene carbon carbon attached to C4 while that at 22.9 δ (20) is due to methylene carbon C5.
A structure for the molecule which meets the spectral descriptions given is

Want to see more full solutions like this?
Chapter 19 Solutions
Study Guide with Student Solutions Manual for McMurry's Organic Chemistry, 9th
- 93 = Volume 93 = 5.32× 10 3 -23 ст a √ 1073 5.32× 10 3 cm³arrow_forwardASP.....arrow_forwardQuestion 7 (10 points) Identify the carboxylic acid present in each of the following items and draw their structures: Food Vinegar Oranges Yogurt Sour Milk Pickles Acid Structure Paragraph ✓ BI UAE 0118 + v Task: 1. Identify the carboxylic acid 2. Provide Name 3. Draw structure 4. Take a picture of your table and insert Add a File Record Audio Record Video 11.arrow_forward
- Check the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Molecule 1 Molecule 2 IZ IN Molecule 4 Molecule 5 ZI none of the above ☐ Molecule 3 Х IN www Molecule 6 NH Garrow_forwardHighlight each chiral center in the following molecule. If there are none, then check the box under the drawing area. There are no chiral centers. Cl Cl Highlightarrow_forwardA student proposes the following two-step synthesis of an ether from an alcohol A: 1. strong base A 2. R Is the student's proposed synthesis likely to work? If you said the proposed synthesis would work, enter the chemical formula or common abbreviation for an appropriate strong base to use in Step 1: If you said the synthesis would work, draw the structure of an alcohol A, and the structure of the additional reagent R needed in Step 2, in the drawing area below. If there's more than one reasonable choice for a good reaction yield, you can draw any of them. ☐ Click and drag to start drawing a structure. Yes No ロ→ロ 0|0 G Х D : ☐ பarrow_forward
- टे Predict the major products of this organic reaction. Be sure to use wedge and dash bonds when necessary, for example to distinguish between different major products. ☐ ☐ : ☐ + NaOH HO 2 Click and drag to start drawing a structure.arrow_forwardShown below are five NMR spectra for five different C6H10O2 compounds. For each spectrum, draw the structure of the compound, and assign the spectrum by labeling H's in your structure (or in a second drawing of the structure) with the chemical shifts of the corresponding signals (which can be estimated to nearest 0.1 ppm). IR information is also provided. As a reminder, a peak near 1700 cm-1 is consistent with the presence of a carbonyl (C=O), and a peak near 3300 cm-1 is consistent with the presence of an O–H. Extra information: For C6H10O2 , there must be either 2 double bonds, or 1 triple bond, or two rings to account for the unsaturation. There is no two rings for this problem. A strong band was observed in the IR at 1717 cm-1arrow_forwardPredict the major products of the organic reaction below. : ☐ + Х ك OH 1. NaH 2. CH₂Br Click and drag to start drawing a structure.arrow_forward
- NG NC 15Show all the steps you would use to synthesize the following products shown below using benzene and any organic reagent 4 carbons or less as your starting material in addition to any inorganic reagents that you have learned. NO 2 NC SO3H NO2 OHarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardShow work...don't give Ai generated solutionarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


