
Concept explainers
(a)
Interpretation: The full name along with the anomeric designation of the following compound should be determined:
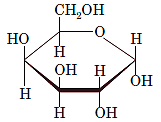
Concept Introduction: A common way of writing structural formula which represent the cyclic structure of monosaccharides is said to be the Haworth projection, which is best represented by chair conformations.
A convention used to represent a 3-D stereo formula in 2-D representation is said to be Fischer projections. In this projection, the vertical lines represent bonds below the plane of the paper and horizontal lines represents bonds above the plane of the paper.
(a)
Answer to Problem 21P
Explanation of Solution
Drawing the Fischer projection of given compound as:
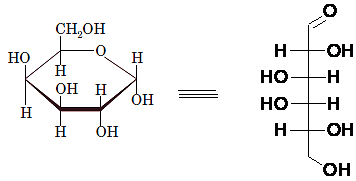
For a D-sugar, the group attached to the bottom chiral carbon is present at the right-hand side whereas for L-sugar it is present at left-hand side.
A new carbon stereocenter created by the conversion of an open structure to a cyclic structure is said to be the anomeric carbon. In carbohydrates, when the hydroxy group on an anomeric carbon is present on the same side as that of the
The anomeric carbon of the given compound is shown by “*”:
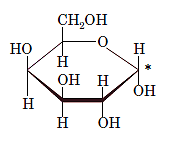
Since, in the given sugar the
Hence, the name of compound is
(b)
Interpretation: The full name along with the anomeric designation of the following compound should be determined:
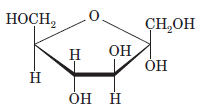
Concept Introduction: A common way of writing structural formula which represent the cyclic structure of monosaccharides is said to be the Haworth projection, which is best represented by chair conformations.
A convention used to represent a 3-D stereo formula in 2-D representation is said to be Fischer projections. In this projection, the vertical lines represent bonds below the plane of the paper and horizontal lines represents bonds above the plane of the paper.
(b)
Answer to Problem 21P
Explanation of Solution
Drawing the Fischer projection of given compound as:
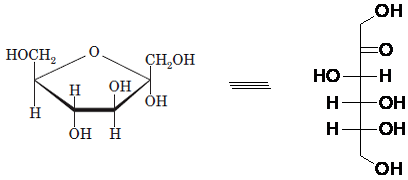
For a D-sugar, the
A new carbon stereocenter created by the conversion of an open structure to a cyclic structure is said to be the anomeric carbon. In carbohydrates, when the hydroxy group on an anomeric carbon is present on the same side as that of the
The anomeric carbon of the given compound is shown by “*”:
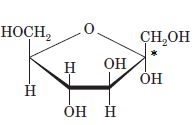
Since, in the given sugar the
Hence, the name of compound is
(c)
Interpretation: The full name along with the anomeric designation of the following compound should be determined:
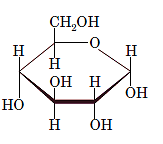
Concept Introduction: A common way of writing structural formula which represent the cyclic structure of monosaccharides is said to be the Haworth projection, which is best represented by chair conformations.
A convention used to represent a 3-D stereo formula in 2-D representation is said to be Fischer projections. In this projection, the vertical lines represent bonds below the plane of the paper and horizontal lines represents bonds above the plane of the paper.
(c)
Answer to Problem 21P
Explanation of Solution
Drawing the Fischer projection of given compound as:
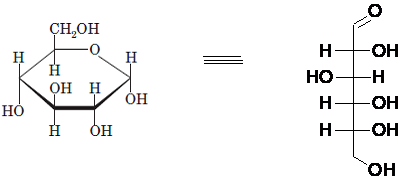
For a D-sugar, the
A new carbon stereocenter created by the conversion of an open structure to a cyclic structure is said to be the anomeric carbon. In carbohydrates, when the hydroxy group on an anomeric carbon is present on the same side as that of the
The anomeric carbon of the given compound is shown by “*”:
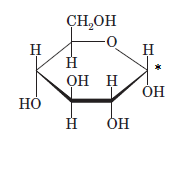
Since, in the given sugar the
Hence, the name of compound is
(d)
Interpretation: The full name along with the anomeric designation of the following compound should be determined:
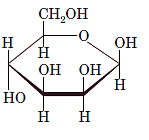
Concept Introduction: A common way of writing structural formula which represent the cyclic structure of monosaccharides is said to be the Haworth projection, which is best represented by chair conformations.
A convention used to represent a 3-D stereo formula in 2-D representation is said to be Fischer projections. In this projection, the vertical lines represent bonds below the plane of the paper and horizontal lines represents bonds above the plane of the paper.
(d)
Answer to Problem 21P
Explanation of Solution
Drawing the Fischer projection of given compound as:
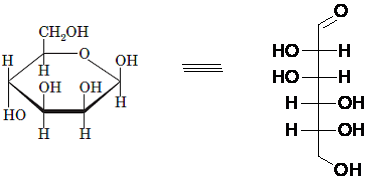
For a D-sugar, the
A new carbon stereocenter created by the conversion of an open structure to a cyclic structure is said to be the anomeric carbon. In carbohydrates, when the hydroxy group on an anomeric carbon is present on the same side as that of the
The anomeric carbon of the given compound is shown by “*”:
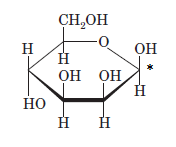
Since, in the given sugar the
Hence, the name of compound is
Want to see more full solutions like this?
Chapter 19 Solutions
Introduction to General, Organic and Biochemistry
- Identify the compound with the longest carbon - nitrogen bond. O CH3CH2CH=NH O CH3CH2NH2 CH3CH2C=N CH3CH=NCH 3 The length of all the carbon-nitrogen bonds are the samearrow_forwardIdentify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forwardH H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forward
- Rank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forwardRank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forwardchoose the description that best describes the geometry for the following charged species ch3-arrow_forward
- Why isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forwardWhat is the approximate bond angle around the nitrogen atom? HNH H Harrow_forwardOH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forward
- Complete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all species within the reaction. S₂O₃²⁻(aq) → S₄O₆²⁻(aq)arrow_forwardQ Select to Edit NH3 (CH3)2CHCI (1 equiv) AICI 3 Select to Draw cat. H2SO4 SO3 (1 equiv) HO SOCl2 pyridine Select to Edit >arrow_forwardComplete and balance the following half-reaction in basic solution. Be sure to include the proper phases for all species within the reaction. Zn(s) → Zn(OH)₄²⁻(aq)arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning





