
General, Organic, & Biological Chemistry
3rd Edition
ISBN: 9780073511245
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 18.8, Problem 18.22P
Interpretation Introduction
Interpretation:
Thechirality center in the given molecule of serotonin needs to be determined.
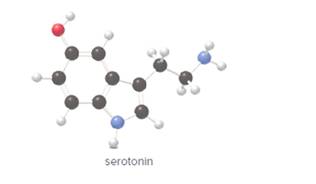
Concept Introduction:
Organic compounds are the compounds which are mainly composed C and H atoms. The branch of chemistry that deals with preparation, reactions, and properties of organic compounds. The molecular formula of organic compound represents the number of bonded atoms with their atomic symbols.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
I need help with the following question
For CARS, which statement is not true regarding its advantages?
a) Contrast signal based on vibrational characteristics, no need for fluorescent tagging.
b) Stronger signals than spontaneous Raman.
c) Suffers from fluorescence interference, because CARS signal is at high frequency.
d) Faster, more efficient imaging for real-time analysis.
e) Higher resolution than spontaneous Raman microscopy.
Draw the major product of the Claisen condensation reaction between two molecules of this ester. Ignore
inorganic byproducts.
Incorrect, 5 attempts remaining
1. NaOCH3/CH3OH
2. Acidic workup
Select to Draw
O
Incorrect, 5 attempts remaining
The total number of carbons in the parent chain is incorrect. Review the reaction conditions including starting materials and/or
intermediate structures and recount the number of carbon atoms in the parent chain of your structure.
OK
Chapter 18 Solutions
General, Organic, & Biological Chemistry
Ch. 18.1 - Prob. 18.1PCh. 18.1 - Prob. 18.2PCh. 18.1 - Prob. 18.3PCh. 18.1 - Prob. 18.4PCh. 18.2 - Prob. 18.5PCh. 18.2 - Prob. 18.6PCh. 18.2 - Prob. 18.7PCh. 18.3 - Prob. 18.8PCh. 18.4 - Decaffeinated coffee is produced by extracting the...Ch. 18.4 - Prob. 18.10P
Ch. 18.5 - Prob. 18.11PCh. 18.5 - Prob. 18.12PCh. 18.5 - Prob. 18.13PCh. 18.6 - Prob. 18.14PCh. 18.6 - Prob. 18.15PCh. 18.6 - Prob. 18.16PCh. 18.6 - Name each ammonium salt. a. ( CH3 NH3)+Cl b. [( CH...Ch. 18.6 - Prob. 18.18PCh. 18.6 - Prob. 18.19PCh. 18.7 - Prob. 18.20PCh. 18.7 - Prob. 18.21PCh. 18.8 - Prob. 18.22PCh. 18.8 - Prob. 18.23PCh. 18.8 - Prob. 18.24PCh. 18.9 - Prob. 18.25PCh. 18.9 - Prob. 18.26PCh. 18.9 - Prob. 18.27PCh. 18.10 - Prob. 18.28PCh. 18 - Prob. 18.29PCh. 18 - Prob. 18.30PCh. 18 - Prob. 18.31PCh. 18 - Prob. 18.32PCh. 18 - Prob. 18.33PCh. 18 - Prob. 18.34PCh. 18 - Prob. 18.35PCh. 18 - Prob. 18.36PCh. 18 - Prob. 18.37PCh. 18 - Prob. 18.38PCh. 18 - Give an acceptable name for each amine. a. b.Ch. 18 - Give an acceptable name for each amine. a. b.Ch. 18 - Give an acceptable name for each amine. a. b. c....Ch. 18 - Give an acceptable name for each amine. a. CH3(...Ch. 18 - Prob. 18.43PCh. 18 - Prob. 18.44PCh. 18 - Prob. 18.45PCh. 18 - Prob. 18.46PCh. 18 - Prob. 18.47PCh. 18 - Prob. 18.48PCh. 18 - Prob. 18.49PCh. 18 - Prob. 18.50PCh. 18 - Which compound in each pair has the higher boiling...Ch. 18 - Which compound in each pair has the higher boiling...Ch. 18 - Draw the hydrogen-bonding interactions that occur...Ch. 18 - Prob. 18.54PCh. 18 - Prob. 18.55PCh. 18 - Which compound has the higher water solubility:...Ch. 18 - Prob. 18.57PCh. 18 - Prob. 18.58PCh. 18 - Draw the products of each acid-base reaction. a....Ch. 18 - Draw the products of each acid-base reaction. a....Ch. 18 - Prob. 18.61PCh. 18 - Prob. 18.62PCh. 18 - What type of nitrogen heterocycle occurs in both...Ch. 18 - Only one of the N atoms in nicotine has a trigonal...Ch. 18 - Prob. 18.65PCh. 18 - Prob. 18.66PCh. 18 - Why are aqueous solutions of an alkaloid slightly...Ch. 18 - Prob. 18.68PCh. 18 - Prob. 18.69PCh. 18 - Explain why patients with Parkinson’s disease...Ch. 18 - Prob. 18.71PCh. 18 - Prob. 18.72PCh. 18 - Prob. 18.73PCh. 18 - Prob. 18.74PCh. 18 - Prob. 18.75PCh. 18 - Prob. 18.76PCh. 18 - Prob. 18.77PCh. 18 - Prob. 18.78PCh. 18 - Prob. 18.79PCh. 18 - Prob. 18.80PCh. 18 - Prob. 18.81PCh. 18 - Prob. 18.82PCh. 18 - Prob. 18.83PCh. 18 - Prob. 18.84PCh. 18 - Prob. 18.85PCh. 18 - Prob. 18.86PCh. 18 - Prob. 18.87PCh. 18 - Why do some antihistamines cause drowsiness while...Ch. 18 - Prob. 18.89PCh. 18 - Prob. 18.90PCh. 18 - Compare the structures of morphine and heroin....Ch. 18 - Prob. 18.92CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using a cell of known pathlength b = 1.25115 x 10-3 cm, a water absorption spectrum was measured. The band at 1645 cm-1, assigned to the O-H bending, showed an absorbance, A, of 1.40. a) Assuming that water density is 1.00 g/mL, calculate the water molar concentration c (hint: M= mole/L) b) Calculate the molar absorptivity, a, of the 1645 cm-1 band c) The transmitted light, I, can be written as I= Ioexp(-xb), where x is the absorption coefficient (sometimes designated as alpha), Io is the input light, and b is the cell pathlength. Prove that x= (ln10)*x*c d) Calculate x for the 1645 cm-1 bandarrow_forwardConvert 1.38 eV into wavelength (nm) and wavenumber (cm-1) (c = 2.998 x 108 m/s; h = 6.626 x 10-34 J*s).arrow_forwardCan you help me understand the CBC method on metal bridging by looking at this problem?arrow_forward
- A partir de Aluminio y Co(NO3)2ꞏ6H2O, indicar las reacciones a realizar para obtener Azul de Thenard (Al2CoO4).arrow_forwardTo obtain Thenard Blue (Al2CoO4), the following reaction is correct (performed in an oven):Al(OH)3 + Co(OH)2 → Al2CoO4 + 4 H2Oarrow_forwardProblem 38 can u explain and solve thanks april 24arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning