
Concept explainers
(a)
Interpretation:
An acceptable name for the following amine should be determined:
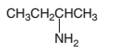
Concept Introduction:
Primary amines are named using the longest carbon chain bonded to the amine nitrogen, and suffix − amine is added at the end of the parent
The naming of secondary and tertiary amines that contains identical alkyl groups are done by using prefix di- or tri- followed by the name of primary amine. Secondary and tertiary amines are containing more than one kind of alkyl group are named as N-substituted primary amines.
Many different nitrogen heterocycles have different names based on ring types and ring size.
(b)
Interpretation:
An acceptable name for the following amine should be determined:
Concept Introduction:
Amines named are based on their structure (
Primary amines are named using the longest carbon chain bonded to the amine nitrogen, and suffix − amine is added at the end of the parent alkane removing the -e of it.
The naming of secondary and tertiary amines that contains identical alkyl groups are done by using prefix di- or tri- followed by the name of primary amine. Secondary and tertiary amines are containing more than one kind of alkyl group are named as N-substituted primary amines.
Many different nitrogen heterocycles have different names based on ring types and ring size.
(c)
Interpretation:
An acceptable name for the following amine should be determined:
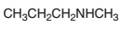
Concept Introduction:
Amines named are based on their structure (
Primary amines are named using the longest carbon chain bonded to the amine nitrogen, and suffix − amine is added at the end of the parent alkane removing the -e of it.
The naming of secondary and tertiary amines that contains identical alkyl groups are done by using prefix di- or tri- followed by the name of primary amine. Secondary and tertiary amines are containing more than one kind of alkyl group are named as N-substituted primary amines.
Many different nitrogen heterocycles have different names based on ring types and ring size.
(d)
Interpretation:
An acceptable name for the following amine should be determined:
Concept Introduction:
Amines named are based on their structure (
Primary amines are named using the longest carbon chain bonded to the amine nitrogen, and suffix − amine is added at the end of the parent alkane removing the -e of it.
The naming of secondary and tertiary amines that contains identical alkyl groups are done by using prefix di- or tri- followed by the name of primary amine. Secondary and tertiary amines are containing more than one kind of alkyl group are named as N-substituted primary amines.
Many different nitrogen heterocycles have different names based on ring types and ring size.
Want to see the full answer?
Check out a sample textbook solution
Chapter 18 Solutions
General, Organic, & Biological Chemistry
- Indicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forwardIndicate the formula of the product obtained by reacting D-Galactose with hydroxylamine.arrow_forward
- helparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning




