
(a)
Interpretation:
From the given compounds from E to H, a compound containing a primary amine and a primary amide needs to be identified.
Concept Introduction:
Amine − is an organic N compounds formed by replacing one or more H atoms of
Amide- is an organic compound contains the group of
Answer to Problem 18.36P
Structure H.
Explanation of Solution
Amines are formed by replacing one or more hydrogen atoms of ammonia with alkyl groups.
Furthermore,
In the below structure, bond that is indicated in red color is the single
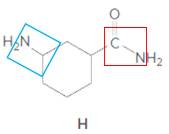
Hence, structure H is a compound that contains a
(b)
Interpretation:
From the given compounds from E to H, a compound containing a primary amine and a secondary amide needs to be identified.
Concept Introduction:
Amine − is an organic N compounds formed by replacing one or more H atoms of
Amide- is an organic compound contains the group of
Answer to Problem 18.36P
Structure F.
Explanation of Solution
Amines are formed by replacing one or more hydrogen atoms of ammonia with alkyl groups.
Furthermore,
In the below structure, bonds that are indicated in red color are the two
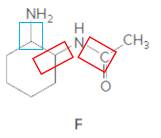
Hence, structure F is a compound that contains a
(c)
Interpretation:
From the given compounds from E to H, a compound containing a secondary amine and a tertiary amide needs to be identified.
Concept Introduction:
Amine − is an organic N compounds formed by replacing one or more H atoms of
Amide- is an organic compound contains the group of
Answer to Problem 18.36P
Structure E.
Explanation of Solution
Amines are formed by replacing one or more hydrogen atoms of ammonia with alkyl groups.
Furthermore,
In the below structure, bonds that are indicated in red color are the three
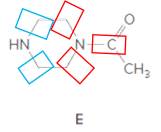
Hence, structure E is a compound that contains a
(d)
Interpretation:
From the given compounds from E to H, a compound containing a tertiary amine and a tertiary amide needs to be identified.
Concept Introduction:
Amine − is an organic N compounds formed by replacing one or more H atoms of
Amide- is an organic compound contains the group of
Answer to Problem 18.36P
Structure G.
Explanation of Solution
Amines are formed by replacing one or more hydrogen atoms of ammonia with alkyl groups.
Furthermore,
In the below structure, bonds that are indicated in red color are the three
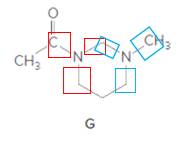
Hence, structure G is a compound that contains a
Want to see more full solutions like this?
Chapter 18 Solutions
General, Organic, & Biological Chemistry
- Polymers may be composed of thousands of monomers. Draw three repeat units (trimer) of the polymer formed in this reaction. Assume there are hydrogen atoms there are hydrogen atoms on the two ends of the trimer. Ignore inorganic byproducts.arrow_forwardDraw a tetramer if this alternating copolymer pleasearrow_forwardDraw the monomers required to synthesize this condensation polymer.arrow_forward
- Draw the monomers required to synthesize this condensation polymer.arrow_forward8:44 PM Sun Apr 13 Earn Freecash.com O Measurement and Matter =1 Setting up a unit conversion 110 Eddie says... ✰ www-awu.aleks.com A student sets up the following equation to convert a measurement. (The ? stands for a number the student is going to calculate.) Fill in the missing part of this equation. Note: your answer should be in the form of one or more fractions multiplied together. (- 4 J kJ -7.0 × 10 ☐ = ? mmol.°C mol °C x10 μ Explanation Check □·□ torox.io Grey Hill LLC. All Rightsarrow_forwardPolymers may be composed of thousands of monomers. Draw three repeat units (trimer) of the polymer formed in this reaction. Assume there are hydrogen atoms there are hydrogen atoms on the two ends of the trimer. Ignore inorganic byproducts please.arrow_forward
- i need help on how to complete the followingarrow_forwardno AI walkthrough current image is wrong answerarrow_forwarda. Determine whether each of the Followery Molecules is in the R- On the y- Configuration 1-01"/ 1-6-4 Br 4 I el Br b. Draw The Fisher projection For all the Meso compounds that can exist FOR The Following molenlearrow_forward

 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

