
Concept explainers
The type of lens should a nearsighted and farsighted person use.
Answer to Problem 33SSC
Nearsighted person should use concave lens
Farsighted person should use convex lens
Explanation of Solution
Introduction:
If two spherical surfaces are joined in such a way that their reflecting surfaces are bent inwardly, the resulting lens is called concave lens. Concave lens deviates the ray of light incident on it and hence it is also called by the name diverging lens.
If two spherical surfaces are joined in such a way that their reflecting surfaces are bent outwardly, the resulting lens is called convex lens. Convex lens is also known as converging lens as it converges the ray of light passes through it and focuses it to a single point on the other side of that lens.
The given figure is shown below.
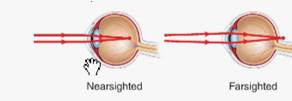
Figure 1
The concave lenses have the ability to reduce the focusing power of the eyes and hence they are commonly used to treat the nearsightedness. It can shift the light rays, focused to a point in front of the retina, backward to the surface of a retina. This resolves the blurred distance vision due to shortsightedness and gives clear vision to a person.
A farsighted person has very weak eye sight and the light rays are focusing behind retina at the backside of eyes as shown in the figure 1. If a farsighted person wear the convex lens, it causes bending of the light rays and helps to focus the light rays on the surface of a retina instead of behind it. Hence gives a clear vision for a farsighted person.
Chapter 18 Solutions
Glencoe Physics: Principles and Problems, Student Edition
Additional Science Textbook Solutions
College Physics: A Strategic Approach (3rd Edition)
Organic Chemistry (8th Edition)
Campbell Biology (11th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Microbiology with Diseases by Body System (5th Edition)
Concepts of Genetics (12th Edition)
- Find the critical angle between ruby and glass. Ruby has n=1.75 and glass has n=1.5Draw an approximate raydiagram for a beam coming 5 degrees less than the critical anglearrow_forwardCalculate the value of the force F at which the 20 kg uniformly dense cabinet will start to tip. Calculate the acceleration of the cabinet at this force F. Must include the FBD and KD of the system. Ignore friction.arrow_forward1) A 2.0 kg toy car travelling along a smooth horizontal surface experiences a horizontal force Fas shown in the picture to the left. Assuming the rightward direction to be positive and if the car has an initial velocity of 60.0m/s to the right, calculate the velocity of the car after the first 10.0s of motion. (Force is in Newtons and time in seconds). (Hint: Use impulse-momentum theorem) F 5.0 10 0 -10arrow_forward
- 3) Two bumper cars of masses 600 kg and 900 kg travelling (on a smooth surface) with velocities 8m/s and 4 m/s respectively, have a head on collision. If the coefficient of restitution is 0.5. a) What sort of collision is this? b) Calculate their velocities immediately after collision. c) If the coefficient of restitution was 1 instead of 0.5, what is the amount of energy lost during collision?arrow_forwardThe rectangular loop of wire shown in the figure (Figure 1) has a mass of 0.18 g per centimeter of length and is pivoted about side ab on a frictionless axis. The current in the wire is 8.5 A in the direction shown. Find the magnitude of the magnetic field parallel to the y-axis that will cause the loop to swing up until its plane makes an angle of 30.0 ∘ with the yz-plane. Find the direction of the magnetic field parallel to the y-axis that will cause the loop to swing up until its plane makes an angle of 30.0 ∘ with the yz-plane.arrow_forwardGive a more general expression for the magnitude of the torque τ. Rewrite the answer found in Part A in terms of the magnitude of the magnetic dipole moment of the current loop m. Define the angle between the vector perpendicular to the plane of the coil and the magnetic field to be ϕ, noting that this angle is the complement of angle θ in Part A. Give your answer in terms of the magnetic moment mm, magnetic field B, and ϕ.arrow_forward
- Calculate the electric and magnetic energy densities at thesurface of a 3-mm diameter copper wire carrying a 15-A current. The resistivity ofcopper is 1.68×10-8 Ω.m.Prob. 18, page 806, Ans: uE= 5.6 10-15 J/m3 uB= 1.6 J/m3arrow_forwardA 15.8-mW laser puts out a narrow beam 2.0 mm in diameter.Suppose that the beam is in free space. What is the rms value of E in the beam? What isthe rms value of B in the beam?Prob. 28, page 834. Ans: Erms= 1380 V/m, Brms =4.59×10-6 Tarrow_forwardA 4.5 cm tall object is placed 26 cm in front of a sphericalmirror. It is desired to produce a virtual image that is upright and 3.5 cm tall.(a) What type of mirror should be used, convex, or concave?(b) Where is the image located?(c) What is the focal length of the mirror?(d) What is the radius of curvature of the mirror?Prob. 25, page 861. Ans: (a) convex, (b) di= -20.2 cm, i.e. 20.2 cm behind the mirror,(c) f= -90.55 cm, (d) r= -181.1 cm.arrow_forward
- A series RCL circuit contains an inductor with inductance L=3.32 mH, and a generator whose rms voltage is 11.2 V. At a resonant frequencyof 1.25 kHz the average power delivered to the circuit is 26.9 W.(a) Find the value of the capacitance.(b) Find the value of the resistance.(c) What is the power factor of this circuit?Ans: C=4.89 μF, R=4.66 Ω, 1.arrow_forwardA group of particles is traveling in a magnetic field of unknown magnitude and direction. You observe that a proton moving at 1.70 km/s in the +x-direction experiences a force of 2.06×10−16 N in the +y-direction, and an electron moving at 4.40 km/s in the −z-direction experiences a force of 8.10×10−16 N in the +y-direction. What is the magnitude of the magnetic force on an electron moving in the −y-direction at 3.70 km/s ? What is the direction of this the magnetic force? (in the xz-plane)arrow_forwardA particle with a charge of −5.20 nC is moving in a uniform magnetic field of B =−( 1.22 T )k^. The magnetic force on the particle is measured to be F=−( 3.50×10−7 N )i^+( 7.60×10−7 N )j^. Calculate the x component of the velocity of the particle.arrow_forward
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON





