
Organic Chemistry
11th Edition
ISBN: 9781118133576
Author: T. W. Graham Solomons, Craig Fryhle
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 18, Problem 29P
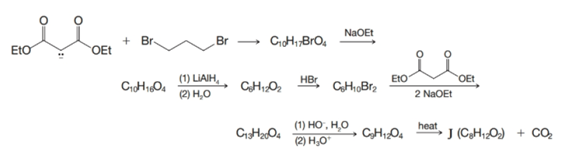
Compound J, a compound with two four-membered rings, has been synthesized by the following route. Outline the steps that are involved.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Highlight each glycosidic bond in the molecule below. Then answer the questions in the table under the drawing area.
HO-
HO-
-0
OH
OH
HO
NG
HO-
HO-
OH
OH
OH
OH
NG
OH
€
+
Suppose the molecule in the drawing area below were reacted with H₂ over a platinum catalyst. Edit the molecule to show what would happen to it. That is, turn
it into the product of the reaction.
Also, write the name of the product molecule under the drawing area.
Name: ☐
H
C=0
X
H-
OH
HO-
H
HO-
-H
CH₂OH
×
Draw the Haworth projection of the disaccharide made by joining D-glucose and D-mannose with a ẞ(1-4) glycosidic bond. If the disaccharide has more than
one anomer, you can draw any of them.
Click and drag to start drawing a
structure.
X
Chapter 18 Solutions
Organic Chemistry
Ch. 18 - Prob. 1PPCh. 18 - Practice Problem 18.2 Would optically active...Ch. 18 - Prob. 3PPCh. 18 - Practice Problem 18.4 Why do we say that the...Ch. 18 - Prob. 5PPCh. 18 - Practice Problem 18.6 (a) Write a reaction...Ch. 18 - PRACTICE PROBLEM 18.7
Show how you would use the...Ch. 18 - Practice Problem 18.8 The acetoacetic ester...Ch. 18 - Practice Problem 18.9
In the synthesis of the keto...Ch. 18 - PRACTICE PROBLEM 18.10 How would you use the...
Ch. 18 - PRACTICE PROBLEM 18.11
How would you use the...Ch. 18 - PRACTICE PROBLEM 18.12 Outline all steps in a...Ch. 18 - PRACTICE PROBLEM 18.13
The antiepileptic drug...Ch. 18 - PRACTICE PROBLEM 18.14 Show how you could employ...Ch. 18 - Prob. 15PCh. 18 - Treating a solution of cis-1-decalone with base...Ch. 18 - Prob. 17PCh. 18 - Prob. 18PCh. 18 - Prob. 19PCh. 18 - Prob. 20PCh. 18 - Prob. 21PCh. 18 - Prob. 22PCh. 18 - Prob. 23PCh. 18 - The synthesis of cyclobutanecarboxylic acid given...Ch. 18 - Prob. 25PCh. 18 - Prob. 26PCh. 18 - Prob. 27PCh. 18 - Prob. 28PCh. 18 - Compound J, a compound with two four-membered...Ch. 18 - Prob. 30PCh. 18 - Prob. 31PCh. 18 - 18.32 Shown below is a synthesis of the elm bark...Ch. 18 - 18.33 (a) A compound U gives a negative iodoform...Ch. 18 - 18.34 Compound A has the molecular formula and...Ch. 18 - Prob. 35PCh. 18 - 1. -Carotene is a highly conjugated hydrocarbon...Ch. 18 - Dehydroabietic acid is a natural product isolated...Ch. 18 - Prob. 1QCh. 18 - Prob. 2QCh. 18 - Prob. 3QCh. 18 - Prob. 4QCh. 18 - Prob. 5Q
Additional Science Textbook Solutions
Find more solutions based on key concepts
In the overhead view of Fig. 9-54, a 300 g ball with a speed v of 6.0 m/s strikes a wall at an angle of 30 and...
Fundamentals of Physics Extended
17. How does the presence of a nonvolatile solute affect the boiling point and melting point of a solution rel...
Introductory Chemistry (6th Edition)
How can the freezing of water crack boulders?
Campbell Biology in Focus (2nd Edition)
Another cross in Drosophila involved the recessive, X-linked genes yellow (y), white (w), and cut (ct). A yello...
Concepts of Genetics (12th Edition)
The accompanying chromosome diagram represents a eukaryotic chromosome prepared with Giemsa stain. Indicate the...
Genetic Analysis: An Integrated Approach (3rd Edition)
11. Birds and mammals are both endothermic, and both have four-chambered hearts. Most reptiles are ectothermic ...
Campbell Biology: Concepts & Connections (9th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Epoxides can be opened in aqueous acid or aqueous base to produce diols (molecules with two OH groups). In this question, you'll explore the mechanism of epoxide opening in aqueous acid. 2nd attempt Be sure to show all four bonds at stereocenters using hash and wedge lines. 0 0 Draw curved arrows to show how the epoxide reacts with hydronium ion. 100 +1: 1st attempt Feedback Be sure to show all four bonds at stereocenters using hash and wedge lines. See Periodic Table See Hint H A 5 F F Hr See Periodic Table See Hintarrow_forward03 Question (1 point) For the reaction below, draw both of the major organic products. Be sure to consider stereochemistry. > 1. CH₂CH₂MgBr 2. H₂O 3rd attempt Draw all four bonds at chiral centers. Draw all stereoisomers formed. Draw the structures here. e 130 AN H See Periodic Table See Hint P C Brarrow_forwardYou may wish to address the following issues in your response if they are pertinent to the reaction(s) you propose to employ:1) Chemoselectivity (why this functional group and not another?) 2) Regioselectivity (why here and not there?) 3) Stereoselectivity (why this stereoisomer?) 4) Changes in oxidation state. Please make it in detail and draw it out too in what step what happens. Thank you for helping me!arrow_forward
- 1) Chemoselectivity (why this functional group and not another?) 2) Regioselectivity (why here and not there?) 3) Stereoselectivity (why this stereoisomer?) 4) Changes in oxidation state. Everything in detail and draw out and write it.arrow_forwardCalculating the pH at equivalence of a titration 3/5 Izabella A chemist titrates 120.0 mL of a 0.7191M dimethylamine ((CH3)2NH) solution with 0.5501 M HBr solution at 25 °C. Calculate the pH at equivalence. The pk of dimethylamine is 3.27. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of HBr solution added. pH = ☐ ✓ 18 Ar Boarrow_forwardAlcohols can be synthesized using an acid-catalyzed hydration of an alkene. An alkene is combined with aqueous acid (e.. sulfuric acid in water). The reaction mechanism typically involves a carbocation intermediate. > 3rd attempt 3343 10 8 Draw arrows to show the reaction between the alkene and hydronium ion. that 2nd attempt Feedback 1st attempt تعمال Ju See Periodic Table See Hint F D Ju See Periodic Table See Hintarrow_forward
- Draw the simplified curved arrow mechanism for the reaction of acetone and CHgLi to give the major product. 4th attempt Π Draw the simplified curved arrow mechanism T 3rd attempt Feedback Ju See Periodic Table See Hint H -H H -I H F See Periodic Table See Hintarrow_forwardSelect the correct reagent to accomplish the first step of this reaction. Then draw a mechanism on the Grignard reagent using curved arrow notation to show how it is converted to the final product. 4th attempt Part 1 (0.5 point) Select the correct reagent to accomplish the first step of this reaction. Choose one: OA Mg in ethanol (EtOH) OB. 2 Li in THF O C. Li in THF D. Mg in THF O E Mg in H2O Part 2 (0.5 point) Br Part 1 Bri Mg CH B CH, 1 Draw intermediate here, but no arrows. © TE See Periodic Table See Hint See Hint ין Harrow_forwardSelect the product for the following reaction. HO HO PCC OH ○ OH O HO ○ HO HO HOarrow_forward
- 5:45 Х Select the final product for the following reaction sequence. O O 1. Mg. ether 2.D.Oarrow_forwardBased on the chart Two similarities between the molecule with alpha glycosidic linkages. Two similarities between the molecules with beta glycosidtic linkages. Two differences between the alpha and beta glycosidic linkages.arrow_forwardplease help fill in the tablearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Characteristic Reactions of Benzene and Phenols; Author: Linda Hanson;https://www.youtube.com/watch?v=tjEqEjDd87E;License: Standard YouTube License, CC-BY
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=-fBPX-4kFlw;License: Standard Youtube License