
Concept explainers
18-4 Answer true or false.
(a) The
(b) The VSEPR model predicts bond angles of 180° about the carbonyl carbon of a carboxyl group.
(c) The VSEPR model predicts bond angles of 109.5° about the oxygen of the OH group of a carboxyl group.
(d) The carbonyl carbon of a carboxyl group can be a stereocenter, depending on its location within a molecule.
(e) Carboxylic acids can be prepared by chromic acid oxidation of primary alcohols and of
(f) The product of chromic acid oxidation of hexanoic acid is 1-hexanol.
(a)
Interpretation:
Whether the statement.
The functional groups of carboxylic acids are a carbonyl group bonded to a hydroxyl group is true or false.
Concept Introduction:
Carboxylic acid group contain one carbonyl group and one hydroxyl group that is −COOH.
Explanation of Solution
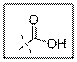
As per the given structure the carboxyl group contains a carbonyl group which is bonded to a hydroxyl group. So the statement is true.
Carboxylic acid functional group is known as COOH. When this group is elaborately written it shows two functional groups. Those groups are carbonyl group and another is hydroxyl group.
So the carboxyl group contains a carbonyl group which is bonded to a hydroxyl group.
(b)
Interpretation:
Whether the statement.
The VSEPR model predicts bond angles of
Concept Introduction:
Carboxylic acid group contain one carbonyl group and one hydroxyl group. VSEPR theory stands for valence shell electron pair repulsion theory. This theory predict the geometry of molecules on the basis of the number of electron pairs present surrounding the central atoms.
Explanation of Solution
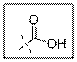
As per the given structure the carboxyl group contains a carbonyl group which is bonded to a hydroxyl group. The angle is
Carboxylic acid functional group is known as COOH. When this group is elaborately written it shows two functional groups. Those groups are carbonyl group and another is hydroxyl group. The hybridization of the carbonyl carbon is sp2 and thus expected bond angle is
(c)
Interpretation:
Whether the statement.
The VSEPR model predicts bond angles of
Concept Introduction:
Carboxylic acid group contain one carbonyl group and one hydroxyl group and the oxygen atom of hydroxyl group is sp3 hybridized. VSEPR theory stands for valence shell electron pair repulsion theory. This theory predict the geometry of molecules on the basis of the number of electron pairs present surrounding the central atoms.
Explanation of Solution
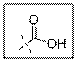
As per the given structure the carboxyl group contains a carbonyl group which is bonded to a hydroxyl group. The oxygen atom of hydroxyl group is sp3 hybridized. So expected bond angle is angle is
But due to two lone pair electrons on oxygen atom bond angle will be lesser than
Carboxylic acid functional group is known as COOH. When this group is elaborately written it shows two functional groups. Those groups are carbonyl group and another is hydroxyl group. The hybridization of the oxygen atom of hydroxyl group is sp3 and thus expected bond angle is
(d)
Interpretation:
Whether the statement.
The carbonyl carbon of a carboxyl group can be a stereocenter, depending on its location within a molecule is true or false.
Concept Introduction:
Carbonyl carbon has double bonded oxygen atom and also linked with hydroxyl group. Stereocentre is defined as the centre in which all the groups attached to the atom generally carbon atom, should be different.
Explanation of Solution
As carbonyl group has double bonded oxygen atom and thus it can’t be a stereocenter. So the statement is false.
Carboxyl group is a carbonyl group bonded to a hydroxyl group. Carbonyl carbon being double bonded to oxygen it is not a stereocenter.
(e)
Interpretation:
Whether the statement.
Carboxylic acid can be prepared by chromic acid oxidation of primary alcohols and of aldehydes is true or false.
Concept Introduction:
Chromic acid is very strong oxidizing agent. Oxidizing agent is defined as the oxidant which gains electrons and reduced in the chemical reaction.
Explanation of Solution
Chromic acid being very strong oxidizing agent it is possible to oxidize primary alcohols and aldehydes to carboxylic acid. So the statement is true.
When primary alcohols are oxidized with chromic acid then the alcohol is first converted to aldehyde which in turn converted to carboxylic acid.
(f)
Interpretation:
Whether the statement.
The product of chromic acid oxidation of hexanoic acid is 1-hexanol is true or false.
Concept Introduction:
When 1-hexanol is oxidized it is converted to hexanoic acid. Oxidation is the process in which either loss of electrons or increase in the oxidation number takes plcae.
Explanation of Solution
1-Hexanol can be converted to hexanoic acid after treatment with chromic acid but the product of chromic acid oxidation of hexanoic acid is not 1-hexanol.
So the statement is false.
1-Hexanol can be converted to hexanoic acid with chromic acid. But 1-hexanol can’t be obtained after oxidizing hexanoic acid with chromic acid. For getting 1-hexanol from hexanoic acid reducing agent is required instead of chromic acid.
Want to see more full solutions like this?
Chapter 18 Solutions
Introduction to General, Organic and Biochemistry
- 6. For each of the following, fill in the synthesis arrows with reagents and show the intermediates. You DO NOT need to use the same number of arrows that are shown (you may use more or less), but the product must be formed from the reactant. Then write the mechanism of one step in the synthesis (you can choose which step to write the mechanism for), including all reagents required, clearly labeling the nucleophile and electrophile for each step, and using curved arrows to show the steps in the mechanism. a. b. OHarrow_forwardDraw the productsarrow_forwardDraw the correct productsarrow_forward
- E Organic Chemistry Maxwell Draw the correct products, in either order, for the ozonolysis reaction: 1) O3, CH2Cl2, -78 °C Product 1 + Product 2 2) Zn, HOAc Draw product 1. Select Draw Templates More C H O presented by M Draw product 2. Erase Select Draw Templates M / # # carrow_forward✓ edict the products of this organic reaction: ---- ။ A CH3–C−NH–CH2–C−CH3 + KOH ? Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. Explanation Check Click anywhere to draw the first atom of your structure. C 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibiliarrow_forwardPredict the product of this organic reaction: A HO-C-CH3 + CH3NH2 P+ H2O Specifically, in the drawing area below draw the condensed structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. Explanation Check Click anywhere to draw the first atom of your structure. marrow_forward
- H 1) OsO4, pyridine 2) Na2SO3 or NaHSO3 in H₂O 2 productsarrow_forward● Biological Macromolecules Naming and drawing cyclic monosaccharides Your answer is incorrect. • Row 1: Your answer is incorrect. Row 3: Your answer is incorrect. • Row 4: Your answer is incorrect. Try again... 0/5 Give the complete common name, including anomer and stereochemistry labels, of the following molecules. You will find helpful information in the ALEKS resource. CH2OH OH OH H H I H OH OH H] H CH2OH H OH ẞ-L-sorbose HOCH2 OH OH H HOCH2 H OH OH H OH H H CH2OH OH H H OH H I- H OH H OH Explanation Recheck W E R % 25 α B Y X & 5 D F G H McGraw Hill LLC. All Rights Reserved. Terms of Use | Pr Parrow_forwardWhat is the missing reactant in this organic reaction? + R -A HO IN + H₂O Specifically, in the drawing area below draw the skeletal ("line") structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. Explanation Check Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forward
- Stuc X ctclix ALE X A ALE אן A ALEX Lab (195 X Nut x M Inb x NU X NUT X Unt x + → C www-awu.aleks.com/alekscgi/x/Isl.exe/10_u-lgNslkr7j8P3jH-IQ1g8NUi-mObKa_ZLx2twjEhK7mVG6PulJI006NcKTV37JxMpZuyrVCdQolLAKqp_7U3r1GUD3... New Chrome available: Naomi Question 26 of 39 (4 points) | Question Attempt: 1 of Unlimited Give the IUPAC name. 2,3-dimethylhexane Part: 1/2 Part 2 of 2 Draw the skeletal structure of a constitutional isomer of the alkane above that contains a different number of carbons in its longest chain. Skip Part Check Click and drag to start drawing a structure. 3 Finance headline Q Search mwa Harvard Intensifi... X Save For Later 00 dlo HB Submit Assignment 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility a 9:11 PM 4/22/2025arrow_forwardPredict the product of this organic reaction: + NH2 HO A P+ H2O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. Click and drag to start drawing a structure. ✓arrow_forward个 Stuc X ctclix ALE X A ALE × A ALE X Lab x (195 × Nut x M Inbx EF 目 → C www-awu.aleks.com/alekscgi/x/Isl.exe/10_u-IgNslkr7j8P3jH-IQ1g8NUi-mObKa_ZLx2twjEhK7mVG6PulJI006NcKTV37JxMpz Chapter 12 HW = Question 27 of 39 (5 points) | Question Attempt: 1 of Unlimited Part: 1/2 Part 2 of 2 Give the IUPAC name. Check 3 50°F Clear ©2025 McGraw Hill L Q Search webp a عالياكarrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co




