
Chemistry: Principles and Reactions
8th Edition
ISBN: 9781305079373
Author: William L. Masterton, Cecile N. Hurley
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 17, Problem 50QAP
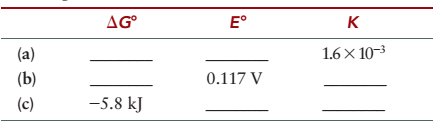
Consider a cell reaction at 25°C where
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
How will you prepare the following buffers? 2.5 L of 1.5M buffer, pH = 10.5 from NH4Cl and NH3
CH₂O
and 22
NMR Solvent: CDCl3
IR Solvent: neat
4000
3000
2000
1500
1000
15
[
اند
6,5
9.8
3.0
7.0
6.0
5.0
4.8
3.0
2.0
1.0
9.8
200
100
protons.
Calculate the mass (in grams) of H3AsO4 (MW=141.9416) needed to produce 3.125 x
1026
Chapter 17 Solutions
Chemistry: Principles and Reactions
Ch. 17 - Balance the following half-equations. Balance (a)...Ch. 17 - Balance the following half-equations. Balance (a)...Ch. 17 - Prob. 3QAPCh. 17 - Balance the following reactions in acid: (a)...Ch. 17 - Write balanced equations for the following...Ch. 17 - Write balanced equations for the following...Ch. 17 - Prob. 7QAPCh. 17 - Write balanced net ionic equations for the...Ch. 17 - Write balanced net ionic equations for the...Ch. 17 - Prob. 10QAP
Ch. 17 - Write a balanced chemical equation for the overall...Ch. 17 - Write a balanced net ionic equation for the...Ch. 17 - Draw a diagram for a salt bridge cell for each of...Ch. 17 - Follow the directions in Question 13 for the...Ch. 17 - Consider a voltaic salt bridge cell represented by...Ch. 17 - Consider a salt bridge voltaic cell represented by...Ch. 17 - Consider a salt bridge cell in which the anode is...Ch. 17 - Follow the directions in Question 17 for a salt...Ch. 17 - Prob. 19QAPCh. 17 - Which species in each pair is the stronger...Ch. 17 - Using Table 17.1, arrange the following reducing...Ch. 17 - Use Table 17.1 to arrange the following oxidizing...Ch. 17 - Consider the following species. Cr3+ Hg(l) H2...Ch. 17 - Follow the directions of Question 23 for the...Ch. 17 - For the following half-reactions, answer these...Ch. 17 - For the following half-reactions, answer the...Ch. 17 - Use Table 17.1 to select (a) a reducing agent in...Ch. 17 - Use Table 17.1 to select (a) an oxidizing agent in...Ch. 17 - Calculate E° for the following voltaic cells: (a)...Ch. 17 - Calculate E° for the following voltaic cells: (a)...Ch. 17 - Using Table 17.1, calculate E° for the reaction...Ch. 17 - Using Table 17.1, calculate E° for the reaction...Ch. 17 - Calculate E° for the following cells: (a)...Ch. 17 - Calculate E° for the following cells: (a)...Ch. 17 - Suppose Ered for Ag+Ag were set equal to zero...Ch. 17 - Suppose Ered for H+H2 were taken to be 0.300 V...Ch. 17 - Which of the following reactions is/are...Ch. 17 - Which of the following reactions is(are)...Ch. 17 - Use the following half-equations to write three...Ch. 17 - Follow the directions of Question 39 for the...Ch. 17 - Use Table 17.1 to answer the following questions:...Ch. 17 - Use Table 17.1 to answer the following questions....Ch. 17 - Write the equation for the reaction, if any, that...Ch. 17 - Write the equation for the reaction, if any, that...Ch. 17 - Prob. 45QAPCh. 17 - Prob. 46QAPCh. 17 - Use Table 17.1 to predict what reaction, if any,...Ch. 17 - Use Table 17.1 to predict what reaction, if any,...Ch. 17 - Consider a cell reaction at 25°C where n=2 . Fill...Ch. 17 - Consider a cell reaction at 25°C where n=4 . Fill...Ch. 17 - For a certain cell, G=25.0 kJ. Calculate E° if n...Ch. 17 - For a certain cell, E=1.08 V. Calculate G° if n is...Ch. 17 - Calculate E°, G°, and K at 25°C for the reaction...Ch. 17 - Calculate E°, G°, and K at 25°C for the reaction...Ch. 17 - Calculate G° at 25°C for each of the reactions...Ch. 17 - Calculate G° at 25°C for each of the reactions...Ch. 17 - Calculate K at 25°C for each of the reactions...Ch. 17 - Calculate K at 25°C for each of the reactions...Ch. 17 - Prob. 59QAPCh. 17 - Use Table 17.1 to find Kffor AuCl4- (aq) at 25°C.Ch. 17 - Prob. 61QAPCh. 17 - What is E° at 25°C for the following reaction?...Ch. 17 - Consider a voltaic cell at 25°C in which the...Ch. 17 - Consider a voltaic cell at 25°C in which the...Ch. 17 - Consider a voltaic cell in which the following...Ch. 17 - Consider a voltaic cell in which the following...Ch. 17 - Calculate the voltages of the following cells at...Ch. 17 - Calculate the voltages of the following cells at...Ch. 17 - Consider the reaction...Ch. 17 - Consider the reaction at 25°C:...Ch. 17 - Complete the following cell notation....Ch. 17 - Complete the following cell notation....Ch. 17 - Consider the reaction below at 25°C:...Ch. 17 - Consider the reaction low at 25°C:...Ch. 17 - Consider a cell in which the reaction is...Ch. 17 - Consider a cell in which the reaction is...Ch. 17 - An electrolytic cell produces aluminum from Al2O3...Ch. 17 - Prob. 78QAPCh. 17 - A solution containing a metal ion (M2+(aq)) is...Ch. 17 - A solution containing a metal ion (M2+(aq)) is...Ch. 17 - A baby's spoon with an area of 6.25 cm2 is plated...Ch. 17 - A metallurgist wants to gold-plate an object with...Ch. 17 - A lead storage battery delivers a current of 6.00...Ch. 17 - Calcium metal can be obtained by the direct...Ch. 17 - Given the following data:...Ch. 17 - In a nickel-cadmium battery (Nicad), cadmium is...Ch. 17 - Hydrogen gas is produced when water is...Ch. 17 - Consider the electrolysis of NiCl2 to Ni(s) and...Ch. 17 - An electrolysis experiment is performed to...Ch. 17 - Prob. 90QAPCh. 17 - Prob. 91QAPCh. 17 - Prob. 92QAPCh. 17 - Atomic masses can be determined by electrolysis....Ch. 17 - Consider the following reaction at 25°C:...Ch. 17 - Given the standard reduction potential for...Ch. 17 - Choose the figure that best represents the results...Ch. 17 - For the cell: Cr|Cr3+Co2+|Co E° is 0.46 V. The...Ch. 17 - Which of the changes below will increase the...Ch. 17 - The standard potential for the reduction of AgSCN...Ch. 17 - Consider the following standard reduction...Ch. 17 - Use Table 17.1 to answer the following questions....Ch. 17 - Consider three metals, X, Y, and Z, and their...Ch. 17 - An alloy made up of tin and copper is prepared by...Ch. 17 - In a fully charged lead storage battery, the...Ch. 17 - Consider a voltaic cell in which the following...Ch. 17 - In biological systems, acetate ion is converted to...Ch. 17 - Consider the cell Pt|H2|H+H+|H2|Pt In the anode...Ch. 17 - Prob. 108QAPCh. 17 - Prob. 109QAP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using what we have learned in CHEM 2310 and up through class on 1/31, propose a series of reaction steps to achieve the transformation below. Be sure to show all reagents and intermediates for full credit. You do not need to draw mechanism arrows, but you do need to include charges where appropriate. If you do not put your group name, you will get half credit at most. ? Brarrow_forwardDraw a mechanism for the formation of 2-bromovanillin using bromonium ion as the reactive electrophile.arrow_forwardNonearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning  Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning
Electrolysis; Author: Tyler DeWitt;https://www.youtube.com/watch?v=dRtSjJCKkIo;License: Standard YouTube License, CC-BY