
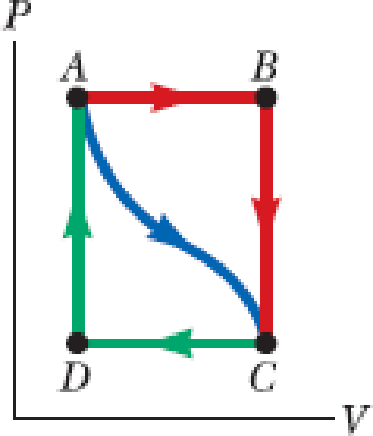
In Figure P17.32, the change in internal energy of a gas that is taken from A to C along the blue path is +800 J. The work done on the gas along the red path ABC is −500 J. (a) How much energy must be added to the system by heat as it goes from A through B to C? (b) If the pressure at point A is five times that of point C, what is the work done on the system in going from C to D? (c) What is the energy exchanged with the surroundings by heat as the gas goes from C to A along the green path? (d) If the change in internal energy in going from point D to point A is +500 J, how much energy must be added to the system by heat as it goes from point C to point D?
Figure P17.32
(a)
The energy added to the system by heat as it goes from
Answer to Problem 32P
The energy added to the system by heat as it goes from
Explanation of Solution
In this case, the change in the internal energy from
Here,
Write the expression for the energy added to the system by heat as it goes from
Here,
Rewrite the above expression,
Conclusion:
Substitute
Therefore, the energy added to the system by heat as it goes from
(b)
The work done on the system from
Answer to Problem 32P
The work done on the system from
Explanation of Solution
Write the expression for the work done the system from
Here,
Substitute
Conclusion:
Substitute
Therefore, the work done on the system from
(c)
The energy exchanged with the surroundings by heat from
Answer to Problem 32P
The energy exchanged with the surroundings by heat from
Explanation of Solution
Write the expression for the work done the system from
Here,
Write the expression for the energy exchanged with the surroundings by heat from
Conclusion:
Substitute
Therefore, the energy exchanged with the surroundings by heat from
(d)
The energy added to the system by heat from point
Answer to Problem 32P
The energy added to the system by heat from point
Explanation of Solution
Write the expression for the energy added to the system by heat as it goes from
Here,
Write the expression for the energy added to the system by heat from point
Here,
Conclusion:
Substitute
Substitute
Therefore, the energy added to the system by heat from point
Want to see more full solutions like this?
Chapter 17 Solutions
Principles of Physics: A Calculus-Based Text
- No chatgpt plsarrow_forwardIn a naval battle, a battleship is attempting to fire on a destroyer. The battleship is a distance d1 = 2,150 m to the east of the peak of a mountain on an island, as shown in the figure below. The destroyer is attempting to evade cannon shells fired from the battleship by hiding on the west side of the island. The initial speed of the shells that the battleship fires is vi = 245 m/s. The peak of the mountain is h = 1,840 m above sea level, and the western shore of the island is a horizontal distance d2 = 250 m from the peak. What are the distances (in m), as measured from the western shore of the island, at which the destroyer will be safe from fire from the battleship? (Note the figure is not to scale. You may assume that the height and width of the destroyer are small compared to d1 and h.)arrow_forwardNo chatgpt plsarrow_forward
- The law of reflection applies to Question 14Select one: a. specular reflection b. irregular reflection c. All of these d. diffuse reflectionarrow_forwardAccording to your book "normal" human body temperature is considered to be ________? Select one: a. none of these b. 98.6°C c. 37°C d. 100°Carrow_forwardProblem Seven. A football receiver running straight downfield at 5.60 m/s is 11.5 m in front of the quarterback when a pass is thrown downfield at an angle of 35.0° above the horizon. 8.) If the receiver never changes speed and the ball is caught at the same height from which it was thrown, find the distance between the quarterback and the receiver when the catch is made. (A) 21.3 (B) 17.8 (C) 18.8 (D) 19.9 (E) 67.5arrow_forward
- When two bar magnets are near each other, the north pole of one of the magnets experiences what type of force from the other magnet? 1. both an attractive force and a repulsive force 2. a Coulomb force 3. only an attractive force 4. only a repulsive forcearrow_forwardWhat can be said about the electric force between two charged particles? It varies as 1/r. It depends only on the magnitudes of the charges. It is much, much greater than the attractive gravitational force. It is repulsive for unlike charges.arrow_forwardA piece of copper originally 305mm long is pulled in tension with a stress of 276MPa. If the deformation is elastic, what will be the resultant elongation. E for copper is 110Gpaarrow_forward
- Please solve and answer the problem correctly please. Be sure to give explanations on each step and write neatly please. Thank you!!arrow_forwardIn the figures, the masses are hung from an elevator ceiling. Assume the velocity of the elevator is constant. Find the tensions in the ropes (in N) for each case. Note that 0₁ = 35.0°, 0₂ = 55.0°, 03 = 60.0°, m₁ = 3.00 kg, and m2 = 7.00 kg. (Due to the nature of this problem, do not use rounded intermediate values-including answers submitted in WebAssign-in your calculations.) (a) Τι WY NY MY T3 e₁ T₁ = N = N = N (b) 18 Τι = Τι T3 = || || || = T T Ts m₂ N N N 02 T₂ T3 m₁arrow_forwardYou are working with a movie director and investigating a scene with a cowboy sliding off a tree limb and falling onto the saddle of a moving horse. The distance of the fall is several meters, and the calculation shows a high probability of injury to the cowboy from the stunt. Let's look at a simpler situation. Suppose the director asks you to have the cowboy step off a platform 2.55 m off the ground and land on his feet on the ground. The cowboy keeps his legs straight as he falls, but then bends at the knees as soon as he touches the ground. This allows the center of mass of his body to move through a distance of 0.660 m before his body comes to rest. (Center of mass will be formally defined in Linear Momentum and Collisions.) You assume this motion to be under constant acceleration of the center of mass of his body. To assess the degree of danger to the cowboy in this stunt, you wish to calculate the average force upward on his body from the ground, as a multiple of the cowboy's…arrow_forward
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
 Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning





