
Principles of Physics: A Calculus-Based Text
5th Edition
ISBN: 9781133104261
Author: Raymond A. Serway, John W. Jewett
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 17, Problem 2P
Consider Joule’s apparatus described in Figure P17.2. The mass of each of the two blocks is 1.50 kg, and the insulated tank is filled with 200 g of water. What is the increase in the water’s temperature after the blocks fall through a distance of 3.00 m?
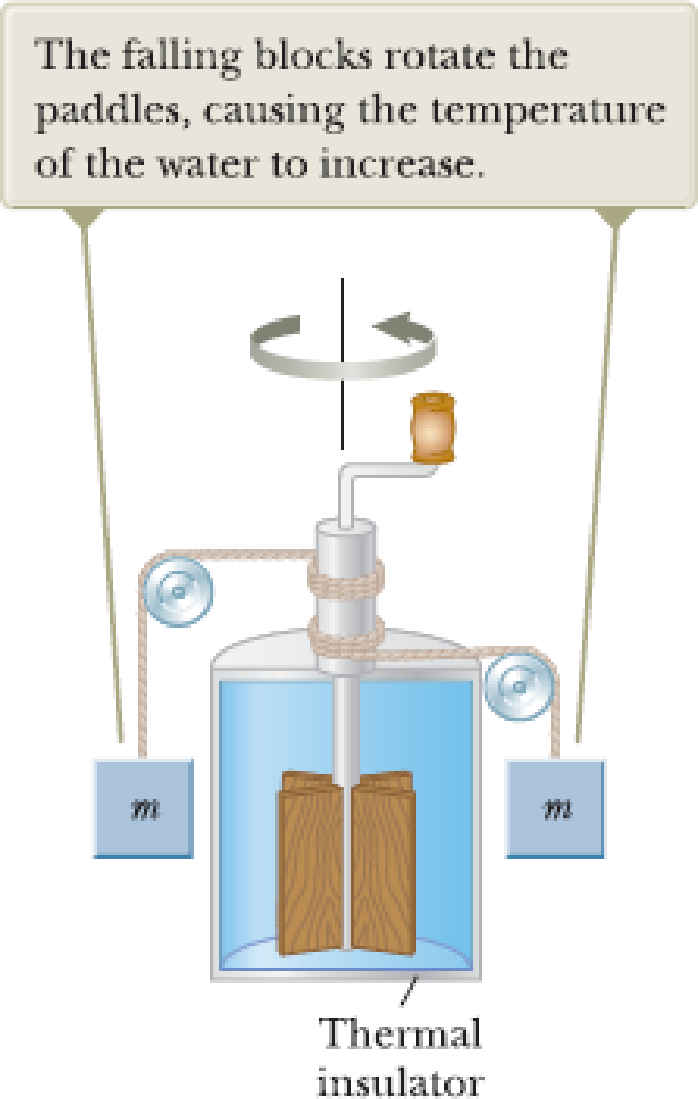
Figure P17.2.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
According to a grade 11 Physics SPH3U course Kinematics, Dynamics, and Energy answer the following question
According to a grade 11 Physics SPH3U course Kinematics, Dynamics, and Energy answer the following question
According to a grade 11 Physics SPH3U course Kinematics, Dynamics, and Energy answer the following question
Chapter 17 Solutions
Principles of Physics: A Calculus-Based Text
Ch. 17.2 - Prob. 17.1QQCh. 17.3 - Prob. 17.2QQCh. 17.3 - Prob. 17.3QQCh. 17.5 - Prob. 17.4QQCh. 17.6 - Characterize the paths in Figure 17.10 as...Ch. 17.7 - (i) How does the internal energy of an ideal gas...Ch. 17.10 - Prob. 17.7QQCh. 17 - Prob. 1OQCh. 17 - A 100-g piece of copper, initially at 95.0C, is...Ch. 17 - Prob. 3OQ
Ch. 17 - Prob. 4OQCh. 17 - Prob. 5OQCh. 17 - Prob. 6OQCh. 17 - Prob. 7OQCh. 17 - Prob. 8OQCh. 17 - Prob. 9OQCh. 17 - Prob. 10OQCh. 17 - Star A has twice the radius and twice the absolute...Ch. 17 - If a gas is compressed isothermally, which of the...Ch. 17 - When a gas undergoes an adiabatic expansion, which...Ch. 17 - Ethyl alcohol has about one-half the specific heat...Ch. 17 - Prob. 15OQCh. 17 - Prob. 1CQCh. 17 - Prob. 2CQCh. 17 - Pioneers stored fruits and vegetables in...Ch. 17 - Why is a person able to remove a piece of dry...Ch. 17 - Prob. 5CQCh. 17 - Prob. 6CQCh. 17 - It is the morning of a day that will become hot....Ch. 17 - You need to pick up a very hot cooking pot in your...Ch. 17 - Rub the palm of your hand on a metal surface for...Ch. 17 - Prob. 10CQCh. 17 - Prob. 11CQCh. 17 - Prob. 12CQCh. 17 - On his honeymoon, James Joule traveled from...Ch. 17 - Consider Joules apparatus described in Figure...Ch. 17 - Prob. 3PCh. 17 - Prob. 4PCh. 17 - Prob. 5PCh. 17 - Prob. 6PCh. 17 - Prob. 7PCh. 17 - Prob. 8PCh. 17 - Prob. 9PCh. 17 - Prob. 10PCh. 17 - Prob. 11PCh. 17 - Prob. 12PCh. 17 - Prob. 13PCh. 17 - Prob. 14PCh. 17 - In an insulated vessel, 250 g of ice at 0C is...Ch. 17 - Prob. 16PCh. 17 - Prob. 17PCh. 17 - Prob. 18PCh. 17 - A 1.00-kg block of copper at 20.0C is dropped into...Ch. 17 - A resting adult of average size converts chemical...Ch. 17 - Prob. 21PCh. 17 - Prob. 22PCh. 17 - An ideal gas is enclosed in a cylinder with a...Ch. 17 - Prob. 24PCh. 17 - Prob. 25PCh. 17 - A sample of an ideal gas goes through the process...Ch. 17 - A thermodynamic system undergoes a process in...Ch. 17 - A gas is taken through the cyclic process...Ch. 17 - Consider the cyclic process depicted in Figure...Ch. 17 - Why is the following situation impossible? An...Ch. 17 - An ideal gas initially at 300 K undergoes an...Ch. 17 - In Figure P17.32, the change in internal energy of...Ch. 17 - Prob. 33PCh. 17 - Prob. 34PCh. 17 - Prob. 35PCh. 17 - Prob. 36PCh. 17 - Prob. 37PCh. 17 - One mole of an ideal gas does 3 000 J of work on...Ch. 17 - A 1.00-mol sample of hydrogen gas is heated at...Ch. 17 - A sample of a diatomic ideal gas has pressure P...Ch. 17 - Prob. 41PCh. 17 - Prob. 42PCh. 17 - Prob. 43PCh. 17 - Review. This problem is a continuation of Problem...Ch. 17 - Prob. 45PCh. 17 - A 2.00-mol sample of a diatomic ideal gas expands...Ch. 17 - Prob. 47PCh. 17 - An ideal gas with specific heat ratio confined to...Ch. 17 - Prob. 49PCh. 17 - Prob. 50PCh. 17 - Prob. 51PCh. 17 - Prob. 52PCh. 17 - Air (a diatomic ideal gas) at 27.0C and...Ch. 17 - Prob. 54PCh. 17 - Prob. 55PCh. 17 - Prob. 56PCh. 17 - Prob. 57PCh. 17 - Prob. 58PCh. 17 - Prob. 59PCh. 17 - Prob. 60PCh. 17 - Prob. 61PCh. 17 - Prob. 62PCh. 17 - The surface of the Sun has a temperature of about...Ch. 17 - Prob. 64PCh. 17 - At high noon, the Sun delivers 1 000 W to each...Ch. 17 - A theoretical atmospheric lapse rate. Section 16.7...Ch. 17 - Prob. 67PCh. 17 - A sample of a monatomic ideal gas occupies 5.00 L...Ch. 17 - An aluminum rod 0.500 m in length and with a...Ch. 17 - Prob. 70PCh. 17 - Prob. 71PCh. 17 - Prob. 72PCh. 17 - Prob. 73PCh. 17 - Prob. 74PCh. 17 - Prob. 75PCh. 17 - Prob. 76PCh. 17 - Prob. 77PCh. 17 - Prob. 78PCh. 17 - Prob. 79PCh. 17 - Prob. 81PCh. 17 - Prob. 82PCh. 17 - Prob. 84PCh. 17 - Prob. 85PCh. 17 - Prob. 86PCh. 17 - Prob. 87PCh. 17 - Prob. 88PCh. 17 - Water in an electric teakettle is boiling. The...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Three point-like charges in the attached image are placed at the corners of an equilateral triangle as shown in the figure. Each side of the triangle has a length of 38.0 cm, and the point (C) is located half way between q1 and q3 along the side. Find the magnitude of the electric field at point (C). Let q1 = −2.80 µC, q2 = −3.40 µC, and q3 = −4.50 µC. Thank you.arrow_forwardThree point-like charges are placed as shown in the attach image, where r1 = r2 = 44.0 cm. Find the magnitude of the electric force exerted on the charge q3. Let q1 = -1.90 uC, q2 = -2.60 uC, and q3 = +3.60 uC. Thank you.arrow_forwardThe drawing attached shows an edge-on view of two planar surfaces that intersect and are mutually perpendicular. Surface (1) has an area of 1.90 m², while Surface (2) has an area of 3.90 m². The electric field in magnitude of 215 N/C. Find the magnitude of the electric flux through surface (1 and 2 combined) if the angle theta made between the electric field with surface (2) is 30.0 degrees. Thank you.arrow_forward
- A car driving at 27m/s veers to the left to avoid a deer in the road. The maneuver takes 2.0s and the direction of travel is altered by 20 degrees. What is the average acceleration during the constant speed maneuver? Do this in accordance with the example in the chapter.arrow_forwardNo No No Chatgpt pls will upvotearrow_forward2 C01: Physical Quantities, Units and Measurementscobris alinu zotinUD TRO Bendemeer Secondary School Secondary Three Express Physics Chpt 1: Physical Quantities, Unit and Measurements Assignment Name: Chen ShiMan loov neowled soria 25 ( 03 ) Class: 3 Respect 6 Date: 2025.01.22 1 Which group consists only of scalar quantities? ABCD A acceleration, moment and energy store distance, temperature and time length, velocity and current mass, force and speed B D. B Which diagram represents the resultant vector of P and Q? lehtele 시 bas siqpeq olarist of beau eldeo qirie-of-qi P A C -B qadmis rle mengaib priwollot erT S Quilons of qira ono mont aboog eed indicator yh from West eril to Inioqbim srij enisinoo MA (6) 08 bas 8A aldao ni nolent or animaleb.gniweb slepe eld 260 km/h D 1 D. e 51arrow_forward
- The figure gives the acceleration a versus time t for a particle moving along an x axis. The a-axis scale is set by as = 12.0 m/s². At t = -2.0 s, the particle's velocity is 11.0 m/s. What is its velocity at t = 6.0 s? a (m/s²) as -2 0 2 t(s) 4arrow_forwardTwo solid cylindrical rods AB and BC are welded together at B and loaded as shown. Knowing that the average normal stress must not exceed 150 MPa in either rod, determine the smallest allowable values of the diameters d₁ and d2. Take P= 85 kN. P 125 kN B 125 kN C 0.9 m 1.2 m The smallest allowable value of the diameter d₁ is The smallest allowable value of the diameter d₂ is mm. mm.arrow_forwardWestros, from Game of Thrones, has an area of approximately 6.73⋅106 miles26.73⋅106miles2. Convert the area of Westros to km2 where 1.00 mile = 1.609 km.arrow_forward
- a) What is the lenght of x? b) Findθ c) Find ϕarrow_forwardA surveyor measures the distance across a straight river by the following method: Starting directly across from a tree on the opposite bank, he walks x = 97.7 m along the riverbank to establish a baseline. Then he sights across to the tree. The angle from his baseline to the tree is θ = 33.0 °. How wide is the river?arrow_forwardA small turtle moves at a speed of 697. furlong/fortnight. Find the speed of the turtle in centimeters per second. Note that 1.00 furlong = 220. yards, 1.00 yard = 3.00 feet, 1.00 foot = 12.0 inches, 1.00 inch = 2.54 cm, and 1.00 fortnight = 14.0 days.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning


College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning
Thermal Expansion and Contraction of Solids, Liquids and Gases; Author: Knowledge Platform;https://www.youtube.com/watch?v=9UtfegG4DU8;License: Standard YouTube License, CC-BY