
(a)
Interpretation:
The products which are formed when following amide is hydrolyzed with water and HCl:
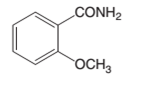
Concept introduction:
Amide has a carbonyl carbon which is linked by a single bond to a nitrogen atom. Amides are
(b)
Interpretation:
The products which are formed when the following amide is hydrolyzed with water and HCl:

Concept introduction:
Amide has a carbonyl carbon which is linked by a single bond to a nitrogen atom. Amides are amine derivatives of carboxylic acids. Hydrolysis of amide can occur in both acidic and basic media..
(c)
Interpretation:
The products which are formed when the following amide is hydrolyzed with water and HCl:
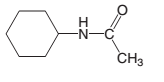
Concept introduction:
Amide has a carbonyl carbon which is linked by a single bond to a nitrogen atom. Amides are amine derivatives of carboxylic acids. Hydrolysis of amide can occur in both acidic and basic media..
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning




