
Concept explainers
What ester is formed when butanoic acid
a.
b.
c.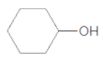
d.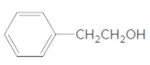
(a)
Interpretation:
The ester formed on treating butanoic acid with CH3OH in the presence of H2SO4 should be determined.
Concept Introduction:
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
Answer to Problem 17.67P
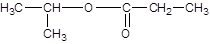
Explanation of Solution
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
The general reaction is written as:
Thus, the reaction between CH3CH2CH2COOH (butanoic acid) and methanol is:
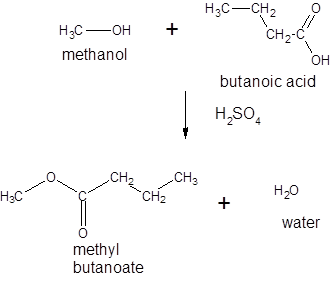
(b)
Interpretation:
The ester formed on treating butanoic acid with CH3CH2CH2OH in the presence of H2SO4 should be determined.
Concept Introduction:
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
Answer to Problem 17.67P
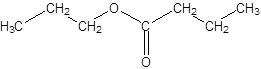
Explanation of Solution
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
The general reaction is written as:
Thus, the reaction between CH3CH2CH2COOH (butanoic acid) and propanol is:
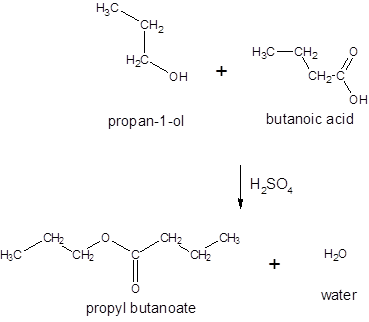
(c)
Interpretation:
The ester formed on treating butanoic acid with following alcohol in the presence of H2SO4 should be determined:
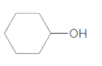
Concept Introduction:
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
Answer to Problem 17.67P
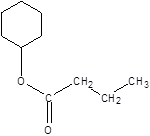
Explanation of Solution
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
The general reaction is written as:
Thus, the reaction between CH3CH2CH2COOH (butanoic acid) and cyclohexanol is:
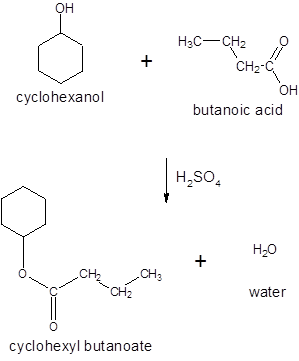
(d)
Interpretation:
The ester formed on treating butanoic acid with following alcohol in the presence of H2SO4 should be determined:
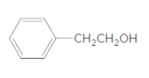
Concept Introduction:
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
Answer to Problem 17.67P
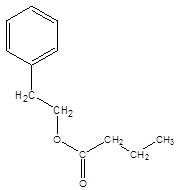
Explanation of Solution
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
The general reaction is written as:
Thus, the reaction between CH3CH2CH2COOH (butanoic acid) and 2-phenylethan-1-ol is:
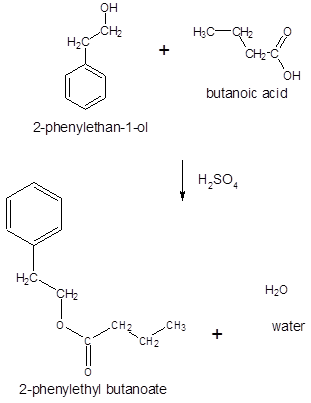
Want to see more full solutions like this?
Chapter 17 Solutions
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
- Draw TWO general chemical equation to prepare Symmetrical and non-Symmetrical ethers Draw 1 chemical reaction of an etherarrow_forwardPlease help me with the following questions for chemistry.arrow_forwardDraw the chemical structure [OR IUPAC name] of the following: a- m-chloromethoxybenzene b.arrow_forward
- Show by chemical equation the reaction of [HCN] and [CH3MgBr] with any alarrow_forwardGive the chemical equation for the preparation of: -Any aldehyde -Any keytonearrow_forward+ C8H16O2 (Fatty acid) + 11 02 → 8 CO2 a. Which of the above are the reactants? b. Which of the above are the products? H2o CO₂ c. Which reactant is the electron donor? Futty acid d. Which reactant is the electron acceptor? e. Which of the product is now reduced? f. Which of the products is now oxidized? 02 #20 102 8 H₂O g. Where was the carbon initially in this chemical reaction and where is it now that it is finished? 2 h. Where were the electrons initially in this chemical reaction and where is it now that it is finished?arrow_forward
- → Acetyl-CoA + 3NAD+ + 1FAD + 1ADP 2CO2 + CoA + 3NADH + 1FADH2 + 1ATP a. Which of the above are the reactants? b. Which of the above are the products? c. Which reactant is the electron donor? d. Which reactants are the electron acceptors? e. Which of the products are now reduced? f. Which product is now oxidized? g. Which process was used to produce the ATP? h. Where was the energy initially in this chemical reaction and where is it now that it is finished? i. Where was the carbon initially in this chemical reaction and where is it now that it is finished? j. Where were the electrons initially in this chemical reaction and where is it now that it is finished?arrow_forwardRank each of the following substituted benzene molecules in order of which will react fastest (1) to slowest (4) by electrophilic aromatic substitution. OCH 3 (Choose one) OH (Choose one) Br (Choose one) Explanation Check NO2 (Choose one) © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Aarrow_forwardFor each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Molecule Inductive Effects O donating O withdrawing O no inductive effects Resonance Effects Overall Electron-Density ○ donating ○ withdrawing O no resonance effects O electron-rich O electron-deficient O similar to benzene Cl O donating O withdrawing ○ donating ○ withdrawing O no inductive effects O no resonance effects O Explanation Check O electron-rich O electron-deficient similar to benzene X © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessarrow_forward
- Identifying electron-donating and For each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Molecule Inductive Effects NH2 ○ donating NO2 Explanation Check withdrawing no inductive effects Resonance Effects Overall Electron-Density ○ donating O withdrawing O no resonance effects O donating O withdrawing O donating withdrawing O no inductive effects Ono resonance effects O electron-rich electron-deficient O similar to benzene O electron-rich O electron-deficient O similar to benzene olo 18 Ar 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forwardRank each of the following substituted benzene molecules in order of which will react fastest (1) to slowest (4) by electrophilic aromatic substitution. Explanation Check Х (Choose one) OH (Choose one) OCH3 (Choose one) OH (Choose one) © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forwardAssign R or S to all the chiral centers in each compound drawn below porat bg 9 Br Brarrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning


