
Concept explainers
Answer the following questions about B, depicted in the ball-and-stick model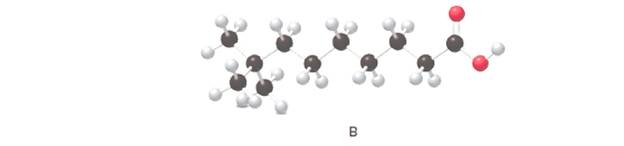
a. What is the IUPAC name for B?
b. Draw an isomer of B that has the same
c. Draw an isomer of B that has a different functional group.
d. What products are formed when B is treated with NaOH?
e. Predict the solubility properties of B in
f. What product is formed when B is treated with
g. What product is formed when B is heated with
(a)
Interpretation:
The IUPAC name for the given ball and stick model should be determined.
Concept Introduction:
Functional groups are the groups of atoms or atoms which are bonded with parent carbon chain in the organic molecule and are responsible for the physical and chemical properties of the compound. In organic chemistry, there are different functional groups such as carboxylic acid, alcohol, ester, or amide.
Answer to Problem 17.92P
8,8-dimethylnonanoic acid.
Explanation of Solution
In the given ball and stick model of the compounds;
Black ball = C atom
White ball = H atom
Red ball = O atom
Blue ball = N atom
To assign the acceptable name to the compound, the IUPAC rules must be followed:
- Check the longest C chain and assign root word for that.
- Add prefix for the branch or side chain with its position.
- Add di, tri, tetra prefix for more than one prefix.
- The primary suffix indicates the single, double and triple bond in the molecule.
- Secondary suffix indicates the presence of functional group in the molecule.
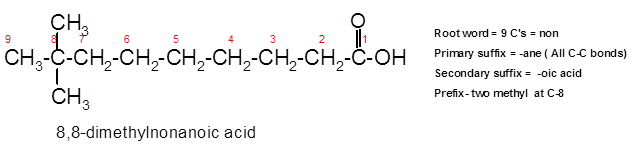
(b)
Interpretation:
The constitutional isomer of 8,8-dimethylnonanoic acid which has same functional group should be determined.
Concept Introduction:
Functional groups are the groups of atoms or atoms which are bonded with parent carbon chain in the organic molecule and are responsible for the physical and chemical properties of the compound. In organic chemistry, there are different functional groups such as carboxylic acid, alcohol, ester, or amide.
Answer to Problem 17.92P
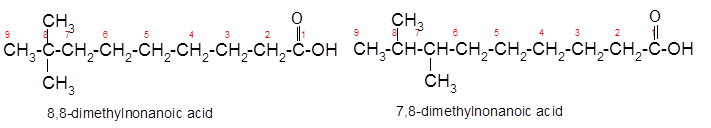
Explanation of Solution
Constitutional isomers are the isomers with same molecular formula but different arrangement of bonded atoms in the molecule. The constitutional isomer of 5-methylhexanoic acid which has same functional group must have same molecular formula but different structural arrangement of bonded atoms.
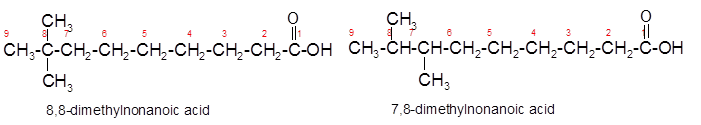
(c)
Interpretation:
The constitutional isomer of 8, 8-dimethylnonanoic acid which has different functional group should be determined.
Concept Introduction:
Functional groups are the groups of atoms or atoms which are bonded with parent carbon chain in the organic molecule and are responsible for the physical and chemical properties of the compound. In organic chemistry, there are different functional groups such as carboxylic acid, alcohol, ester, or amide.
Answer to Problem 17.92P
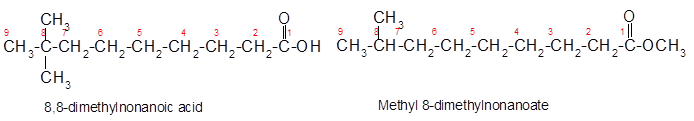
Explanation of Solution
Constitutional isomers are the isomers with same molecular formula but different arrangement of bonded atoms in the molecule. The constitutional isomer of 8, 8-dimethylnonanoic acid which has different functional group must have same molecular formula but different functional group like ester as ester and carboxylic acid are functional isomers of each other.
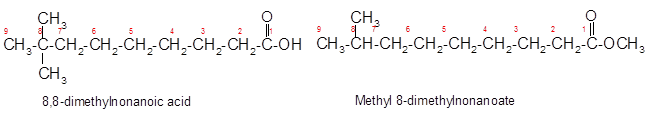
(d)
Interpretation:
The products formed when the given carboxylic 8, 8-dimethylnonanoic acid react with NaOH should be determined.
Concept Introduction:
Functional groups are the groups of atoms or atoms which are bonded with parent carbon chain in the organic molecule and are responsible for the physical and chemical properties of the compound. In organic chemistry, there are different functional groups such as carboxylic acid, alcohol, ester, or amide.
Amines are the organic compounds with general chemical formula of R-NH2 or R-NH-R whereas carboxylic acids are the organic molecules with R-COOH as general chemical formula.
Answer to Problem 17.92P
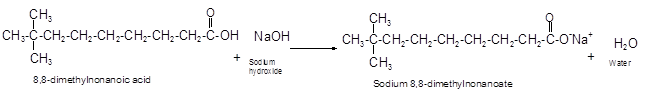
Explanation of Solution
The reaction of carboxylic acid with base like NaOH is an acid-base reaction that leads to the formation of salt and water.
It is also called as neutralization reaction. In these reactions the carboxylic acid gives H+ ions that combines with OH- ion from base and forms water. The carboxylate ion converts to sodium salt due to presence of Na+ ions in the solution.
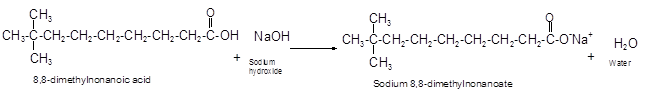
(e)
Interpretation:
The solubility of 8, 8-dimethylnonanoic acid in water and organic solvent should be predicted.
Concept Introduction:
Functional groups are the groups of atoms or atoms which are bonded with parent carbon chain in the organic molecule and are responsible for the physical and chemical properties of the compound. In organic chemistry, there are different functional groups such as carboxylic acid, alcohol, ester, or amide.
Answer to Problem 17.92P
8, 8-dimethylnonanoic acid is less soluble in water but soluble in organic solvents.
Explanation of Solution
Organic compounds like hydrocarbons are composed of C and H atoms. They mainly have C-C and C-H bonds in their structure.
Since both C-C and C-H bonds are non-polar in nature therefore hydrocarbons like alkanes are non-polar compounds therefore it is soluble in non-polar solvents like organic solvents.
The 8, 8-dimethylnonanoic acid is a polar compound due to −COOH group and must form hydrogen bonds with water molecule but due to bulky alkyl group in the molecule it is very difficult for the molecule to form hydrogen bonds with water molecule. Therefore 8, 8-dimethylnonanoic acid is insoluble in water and soluble in organic solvents.
(f)
Interpretation:
The reaction of 8, 8-dimethylnonanoic acid with ethanol in the presence of H2SO4 should be determined.
Concept Introduction:
Functional groups are the groups of atoms or atoms which are bonded with parent carbon chain in the organic molecule and are responsible for the physical and chemical properties of the compound. In organic chemistry, there are different functional groups such as carboxylic acid, alcohol, ester, or amide.
Alcohols are the organic compounds with general chemical formula of R-OH whereas carboxylic acids are the organic molecules with R-COOH as general chemical formula.
Answer to Problem 17.92P
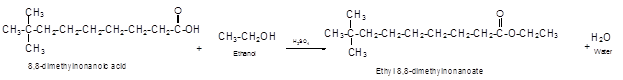
Explanation of Solution
The German chemist Emil Fischer purposed the reaction of carboxylic acid with alcohol in acidic medium to form ester and water. The reaction occurs in the presence of concentrated sulfuric acid. In this reaction the alcohol carbon atom react with carbonyl carbon atom of carboxylic acid to form ester.
The reaction of 8, 8-dimethylnonanoic acid with ethanol leads to formation of water and ester that must have −COO- group in the molecule.
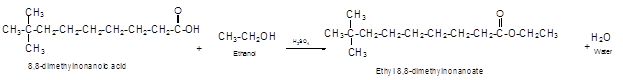
(g)
Interpretation:
The reaction of 8, 8-dimethylnonanoic acid with ethylamine should be determined.
Concept Introduction:
Functional groups are the groups of atoms or atoms which are bonded with parent carbon chain in the organic molecule and are responsible for the physical and chemical properties of the compound. In organic chemistry, there are different functional groups such as carboxylic acid, alcohol, ester, or amide.
Alcohols are the organic compounds with general chemical formula of R-OH whereas carboxylic acids are the organic molecules with R-COOH as general chemical formula.
Answer to Problem 17.92P
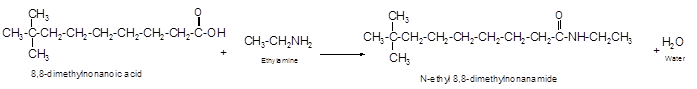
Explanation of Solution
The reaction of carboxylic acid with ammonia or amines forms amide molecules. It involves the formation of water molecule. In this reaction the amine nitrogen atom react with carbonyl carbon atom of carboxylic acid to form amide.
The reaction of 8, 8-dimethylnonanoic acid with ethylamine leads to formation of water and amide that must have −CONH- group in the molecule.
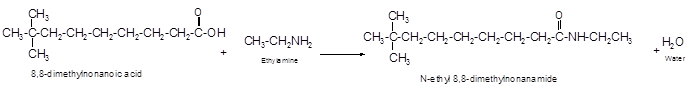
Want to see more full solutions like this?
Chapter 17 Solutions
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
- For which reaction below does the enthalpy change under standard conditions correspond to a standard enthalpy of formation? (Choose all that applies) SO2(g) + 1/2 O2(g) → SO3(g) 2H2(g) + C(s) → CH4(g) Mg(s) + 1/2 O2(g) → MgO(s) CO(g) + H2O(g) → CO2(g) + H2(g) CO2(g) + H2(g) → CO(g) + H2O(g) 1/2 H2(g) + 1/2 N2(g) + 3/2 O2(g) → HNO3(g) CO2(g) + C(s) 2CO(g) N2(g) + 202(g) → 2NO2(g)arrow_forwardChoose all the molecules with zero standard-enthalpy-of-formation (AH% = 0) Fe(s) FeCl2(s) N2(g) H2O(l) 02(g) C(graphite) K(s) H2O(g)arrow_forward8.5 g of potassium hydroxide (molar mass = 56.1 g/mol) dissolves in 125 g of water and the temperature of the solution increases by 15.58°C. Calculate the AH soln for potassium hydroxide. Assume the specific heat capacity of the solution is 4.2 J.g¨¹.ºC-1. KOH(s) → →K+ K(aq) + OH AH solution = ?kJ/mol (aq)arrow_forward
- What will be the final temperature of a 8.79 g piece of iron (CP = 25.09 J/(mol · oC)) initially at 25.0oC, if it is supplied with 302.8 J from a stove?arrow_forwardIdentify the set of stoichiometric coefficients that balances the reaction equation for the combustion of the hydrocarbon below: _ C19 H4002 → CO2 + H2Oarrow_forwardThe cooling system in an automobile holds 11.3 L of ethylene glycol antifreeze. How much energy is absorbed when the temperature of the ethylene glycol goes from 20oC to 100oC? The density and specific heat capacity of ethylene glycol are 1.11 g/mL and 2.42 J/(g ⋅ oC), respectively.arrow_forward
- Which statement about the following chemical reaction is not correct? 2NH3+202 →→→ N2O + 3H₂O ○ It requires 2 mol of ammonia to produce 3 mol of water. It requires 2 mol of dioxygen to produce 1 mol of N2O. ○ Nine moles of water are produced when four moles of ammonia are consumed. Two moles of N2O would be produced when four moles of dioxygen are consumed. Two moles of ammonia react with two moles of dioxygen.arrow_forwardIf 169.7 g of NaOH (40.0 g/mol) were used to prepare 3411.0 mL of solution, what would the concentration be? Group of answer choicesarrow_forwardThe mass of 3.6 mol of some element is 576 g. What is the element?arrow_forward
- I have a question about this problem involving mechanisms and drawing curved arrows for acids and bases. I know we need to identify the nucleophile and electrophile, but are there different types of reactions? For instance, what about Grignard reagents and other types that I might not be familiar with? Can you help me with this? I want to identify the names of the mechanisms for problems 1-14, such as Gilman reagents and others. Are they all the same? Also, could you rewrite it so I can better understand? The handwriting is pretty cluttered. Additionally, I need to label the nucleophile and electrophile, but my main concern is whether those reactions differ, like the "Brønsted-Lowry acid-base mechanism, Lewis acid-base mechanism, acid-catalyzed mechanisms, acid-catalyzed reactions, base-catalyzed reactions, nucleophilic substitution mechanisms (SN1 and SN2), elimination reactions (E1 and E2), organometallic mechanisms, and so forth."arrow_forwardI have a question about this problem involving mechanisms and drawing curved arrows for acids and bases. I know we need to identify the nucleophile and electrophile, but are there different types of reactions? For instance, what about Grignard reagents and other types that I might not be familiar with? Can you help me with this? I want to identify the names of the mechanisms for problems 1-14, such as Gilman reagents and others. Are they all the same? Also, could you rewrite it so I can better understand? The handwriting is pretty cluttered. Additionally, I need to label the nucleophile and electrophile, but my main concern is whether those reactions differ, like the "Brønsted-Lowry acid-base mechanism, Lewis acid-base mechanism, acid-catalyzed mechanisms, acid-catalyzed reactions, base-catalyzed reactions, nucleophilic substitution mechanisms (SN1 and SN2), elimination reactions (E1 and E2), organometallic mechanisms, and so forth."arrow_forwardI have a question about this problem involving mechanisms and drawing curved arrows for acids and bases. I know we need to identify the nucleophile and electrophile, but are there different types of reactions? For instance, what about Grignard reagents and other types that I might not be familiar with? Can you help me with this? I want to identify the names of the mechanisms for problems 1-14, such as Gilman reagents and others. Are they all the same? Also, could you rewrite it so I can better understand? The handwriting is pretty cluttered. Additionally, I need to label the nucleophile and electrophile, but my main concern is whether those reactions differ, like the "Brønsted-Lowry acid-base mechanism, Lewis acid-base mechanism, acid-catalyzed mechanisms, acid-catalyzed reactions, base-catalyzed reactions, nucleophilic substitution mechanisms (SN1 and SN2), elimination reactions (E1 and E2), organometallic mechanisms, and so forth."arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
