
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
3rd Edition
ISBN: 9781259298424
Author: SMITH
Publisher: VST
expand_more
expand_more
format_list_bulleted
Question
Chapter 17, Problem 17.76P
Interpretation Introduction
(a)
Interpretation:
The
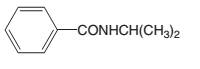
Concept introduction:
There are different methods to synthesize amides. The reaction between a carboxylic acid and an amine is one method.
Interpretation Introduction
(b)
Interpretation:
The carboxylic acid and amine which are needed to synthesis the following compound should be determined:
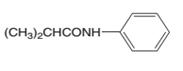
Concept introduction:
There are different methods to synthesize amides. The reaction between a carboxylic acid and an amine is one method.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1) Calculate the longest and shortest wavelengths in the Lyman and Paschen series.
2) Calculate the ionization energy of He* and L2+ ions in their ground states.
3) Calculate the kinetic energy of the electron emitted upon irradiation of a H-atom in ground state by a 50-nm radiation.
Calculate the ionization energy of He+ and Li²+ ions in their ground states.
Thannnxxxxx sirrr
Ahehehehehejh27278283-4;*; shebehebbw $+$;$-;$-28283773838 hahhehdva
Plleeaasseee solllveeee question 3 andd thankss sirr, don't solve it by AI plleeaasseee don't use AI
Chapter 17 Solutions
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
Ch. 17.1 - Draw out each compound to clearly show what groups...Ch. 17.1 - Prob. 17.2PCh. 17.1 - (a) Label each functional group in lisinopril, a...Ch. 17.2 - Give the IUPAC name for each compound. a. b. c.Ch. 17.2 - Give the structure corresponding to each IUPAC...Ch. 17.2 - Prob. 17.6PCh. 17.2 - Prob. 17.7PCh. 17.2 - Prob. 17.8PCh. 17.2 - Prob. 17.9PCh. 17.2 - Prob. 17.10P
Ch. 17.2 - Prob. 17.11PCh. 17.3 - Which compound in each pair has the higher boiling...Ch. 17.4 - Prob. 17.13PCh. 17.5 - In addition to ethyl butanoate (Section 17.5),...Ch. 17.6 - Prob. 17.15PCh. 17.6 - Prob. 17.16PCh. 17.6 - Prob. 17.17PCh. 17.6 - Prob. 17.18PCh. 17.6 - Prob. 17.19PCh. 17.7 - Prob. 17.20PCh. 17.7 - Ibuprofen is another pain reliever that is a...Ch. 17.8 - Prob. 17.22PCh. 17.8 - Prob. 17.23PCh. 17.8 - Prob. 17.24PCh. 17.8 - Prob. 17.25PCh. 17.8 - Prob. 17.26PCh. 17.9 - Prob. 17.27PCh. 17.9 - Prob. 17.28PCh. 17.9 - Prob. 17.29PCh. 17.9 - Prob. 17.30PCh. 17.9 - Prob. 17.31PCh. 17.9 - Prob. 17.32PCh. 17.10 - Prob. 17.33PCh. 17.10 - Prob. 17.34PCh. 17.10 - Prob. 17.35PCh. 17.11 - Prob. 17.36PCh. 17 - Prob. 17.37PCh. 17 - Prob. 17.38PCh. 17 - Prob. 17.39PCh. 17 - Prob. 17.40PCh. 17 - Prob. 17.41PCh. 17 - Prob. 17.42PCh. 17 - Prob. 17.43PCh. 17 - Prob. 17.44PCh. 17 - Prob. 17.45PCh. 17 - Prob. 17.46PCh. 17 - Prob. 17.47PCh. 17 - Give an acceptable name for each ester. a. CH3CO2(...Ch. 17 - Prob. 17.49PCh. 17 - Prob. 17.50PCh. 17 - Prob. 17.51PCh. 17 - Give an acceptable name for each compound.
a.
b....Ch. 17 - Prob. 17.53PCh. 17 - Draw the structure corresponding to each name. a....Ch. 17 - Draw the structure corresponding to each name. a....Ch. 17 - Draw the structure corresponding to each name. a....Ch. 17 - Prob. 17.57PCh. 17 - Prob. 17.58PCh. 17 - Prob. 17.59PCh. 17 - Prob. 17.60PCh. 17 - Prob. 17.61PCh. 17 - Prob. 17.62PCh. 17 - Prob. 17.63PCh. 17 - Prob. 17.64PCh. 17 - Prob. 17.65PCh. 17 - Prob. 17.66PCh. 17 - What ester is formed when butanoic acid...Ch. 17 - Prob. 17.68PCh. 17 - Prob. 17.69PCh. 17 - Prob. 17.70PCh. 17 - Prob. 17.71PCh. 17 - Prob. 17.72PCh. 17 - Prob. 17.73PCh. 17 - Prob. 17.74PCh. 17 - Prob. 17.75PCh. 17 - Prob. 17.76PCh. 17 - Prob. 17.77PCh. 17 - Prob. 17.78PCh. 17 - Prob. 17.79PCh. 17 - Prob. 17.80PCh. 17 - Prob. 17.81PCh. 17 - Prob. 17.82PCh. 17 - Prob. 17.83PCh. 17 - Prob. 17.84PCh. 17 - Prob. 17.85PCh. 17 - Prob. 17.86PCh. 17 - What is the difference between saponification and...Ch. 17 - You have now learned three different types of...Ch. 17 - Draw the products formed in each reaction.
Ch. 17 - Prob. 17.90PCh. 17 - Answer the following questions about A, depicted...Ch. 17 - Answer the following questions about B, depicted...Ch. 17 - Prob. 17.93PCh. 17 - Prob. 17.94PCh. 17 - Prob. 17.95PCh. 17 - Prob. 17.96PCh. 17 - Prob. 17.97PCh. 17 - Prob. 17.98PCh. 17 - Prob. 17.99PCh. 17 - Prob. 17.100PCh. 17 - Prob. 17.101PCh. 17 - Prob. 17.102PCh. 17 - Prob. 17.103CPCh. 17 - Lactams can be hydrolyzed with base, just like...
Knowledge Booster
Similar questions
- Calculate the chemical shifts in 13C and 1H NMR for 4-chloropropiophenone ? Write structure and label hydrogens and carbonsarrow_forwardPlease sirrr soollveee these parts pleaseeee and thank youuuuuarrow_forwardPlease sirrr soollveee these parts pleaseeee and thank youuuuu, don't solve it by AI plleeaasseeearrow_forward
- Please sirrr soollveee these parts pleaseeee and thank youuuuuarrow_forward4. Read paragraph 4.15 from your textbook, use your calculated lattice energy values for CuO, CuCO3 and Cu(OH)2 an explain thermal decomposition reaction of malachite: Cu2CO3(OH)2 →2CuO + H2O + CO2 (3 points)arrow_forwardPlease sirrr soollveee these parts pleaseeee and thank youuuuuarrow_forward
- III O Organic Chemistry Using wedges and dashes in skeletal structures Draw a skeletal ("line") structure for each of the molecules below. Be sure your structures show the important difference between the molecules. key O O O O O CHON Cl jiii iiiiiiii You can drag the slider to rotate the molecules. Explanation Check Click and drag to start drawing a structure. Q Search X G ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use F 3 W C 3/5arrow_forward3. Use Kapustinskii's equation and data from Table 4.10 in your textbook to calculate lattice energies of Cu(OH)2 and CuCO3 (4 points)arrow_forward2. Copper (II) oxide crystalizes in monoclinic unit cell (included below; blue spheres 2+ represent Cu²+, red - O²-). Use Kapustinski's equation (4.5) to calculate lattice energy for CuO. You will need some data from Resource section of your textbook (p.901). (4 points) CuOarrow_forward
- What is the IUPAC name of the following compound? OH (2S, 4R)-4-chloropentan-2-ol O (2R, 4R)-4-chloropentan-2-ol O (2R, 4S)-4-chloropentan-2-ol O(2S, 4S)-4-chloropentan-2-olarrow_forwardIn the answer box, type the number of maximum stereoisomers possible for the following compound. A H H COH OH = H C Br H.C OH CHarrow_forwardSelect the major product of the following reaction. Br Br₂, light D Br Br Br Brarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,