
Concept explainers
(a)
Interpretation:
The acceptable name for the following
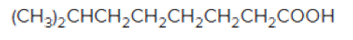
Concept introduction:
An organic compound in which carboxy functional group that is -COOH is bonded to the carbon atom is said to be a carboxylic acid. The general formula for carboxylic acid is RCOOH or RCO2H.
In order to give the name to the carboxylic acid group, the following steps are followed:
1. The parent (longest)
2. The ending of the parent chain from alkane (-e) is changed to -oic acid for a carboxylic acid group.
3. The numbering is of the chain is done in such a way that carbonyl carbon gets the smaller number.
4. Name should be written in alphabetical order and other substituents are shown by the number.
For number of carbons atoms chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
(b)
Interpretation:
The acceptable name for the following carboxylic acid should be determined:
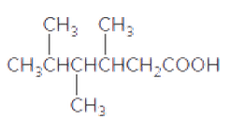
Concept introduction:
An organic compound in which carboxy functional group that is -COOH is bonded to the carbon atom is said to be a carboxylic acid. The general formula for carboxylic acid is RCOOH or RCO2H.
In order to give the name to the carboxylic acid group, the following steps are followed:
1. The parent (longest) alkane chain is identified.
2. The ending of the parent chain from alkane (-e) is changed to -oic acid for a carboxylic acid group.
3. The numbering is of the chain is done in such a way that carbonyl carbon gets the smaller number.
4. Name should be written in alphabetical order and other substituents are shown by the number.
For number of carbons atoms chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
(c)
Interpretation:
The acceptable name for the following carboxylic acid should be determined:
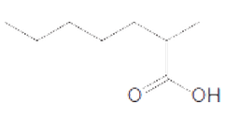
Concept introduction:
An organic compound in which carboxy functional group that is -COOH is bonded to the carbon atom is said to be a carboxylic acid. The general formula for carboxylic acid is RCOOH or RCO2H.
In order to give the name to the carboxylic acid group, the following steps are followed:
1. The parent (longest) alkane chain is identified.
2. The ending of the parent chain from alkane (-e) is changed to -oic acid for a carboxylic acid group.
3. The numbering is of the chain is done in such a way that carbonyl carbon gets the smaller number.
4. Name should be written in alphabetical order and other substituents are shown by the number.
For number of carbons atoms chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
General, Organic, & Biological Chemistry
- An einstein is the amount of energy needed to dissociate 1 mole of a substance. If we have 0.58 moles, do we need 0.58 einsteins to dissociate that substance?arrow_forwardIf the energy absorbed per mole of gas is 480 kJ mol-1, indicate the number of Einsteins per mole.Data: Energy of each photon: 0.7835x10-18 J.arrow_forwardIf the energy absorbed per mole of gas is 480 kJ mol-1, indicate the number of Einsteins per mole.arrow_forward
- The quantum yield of the photochemical decay of HI is 2. Calculating the moles of HI per kJ of radiant energy can be decayed knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forwardThe quantum yield of the photochemical decay of HI is 2. Calculate the number of Einsteins absorbed per mole knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forwardThe quantum yield of the photochemical decay of HI is 2. How many moles of HI per kJ of radiant energy can be decayed knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forward
- If the energy absorbed per mole of photons is 450 kJ, the number of Einsteins absorbed per 1 mole.arrow_forwardWhen propionic aldehyde in vapor form at 200 mmHg and 30°C is irradiated with radiation of wavelength 302 nm, the quantum yield with respect to the formation of CO is 0.54. If the intensity of the incident radiation is 1.5x10-3 W, find the rate of formation of CO.arrow_forwardDraw mechanismarrow_forward
- Does Avogadro's number have units?arrow_forwardExplain why the total E in an Einstein depends on the frequency or wavelength of the light.arrow_forwardIf the dissociation energy of one mole of O2 is 5.17 eV, determine the wavelength that must be used to dissociate it with electromagnetic radiation. Indicate how many Einstein's of this radiation are needed to dissociate 1 liter of O2 at 25°C and 1 atm of pressure.Data: 1 eV = 96485 kJ mol-1; R = 0.082 atm L K-1; c = 2.998x108 m s-1; h = 6.626x10-34 J s; NA = 6.022x 1023 mol-1arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

