
(a)
Interpretation:
The given amine has to be classified as primary, secondary, or tertiary amine.
Concept Introduction:
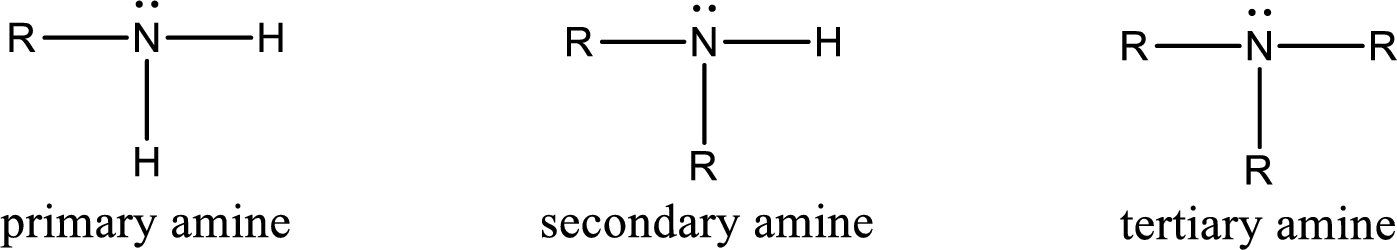
Amine is an organic derivative. If in ammonia one or more alkyl, cycloalkyl, or aryl groups are substituted instead of hydrogen atom then it is known as amine. Depending on the number of substitution the
Amides are also organic derivative. In an amide, the nitrogen atom is bonded to a carbonyl group. The general structural formula of amide can be given as shown below,
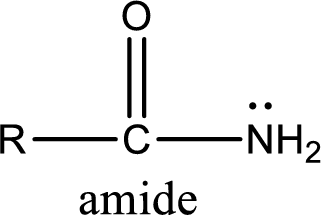
The difference between amine and amide is that in amine, the nitrogen atom is bonded to a hydrocarbon chain. In case of amides, the nitrogen atom is bonded to a carbonyl group.
(b)
Interpretation:
The given amine has to be classified as primary, secondary, or tertiary amine.
Concept Introduction:
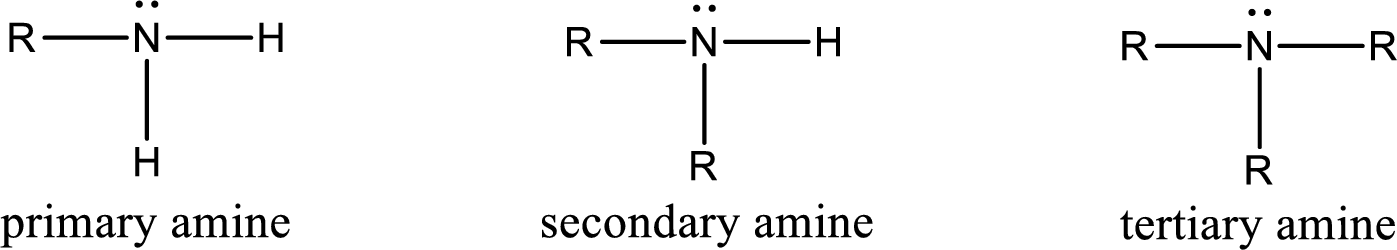
Amine is an organic derivative. If in ammonia one or more alkyl, cycloalkyl, or aryl groups are substituted instead of hydrogen atom then it is known as amine. Depending on the number of substitution the amines are classified as primary, secondary or tertiary amine. Primary amine is the one in which only one hydrogen atom in ammonia is replaced by a hydrocarbon group. Secondary amine is the one in which only two hydrogen atoms in ammonia is replaced by a hydrocarbon group. Tertiary amine is the one in which all three hydrogen atoms in ammonia is replaced by a hydrocarbon group. The generalized structural formula for all the amines is,
Amides are also organic derivative. In an amide, the nitrogen atom is bonded to a carbonyl group. The general structural formula of amide can be given as shown below,
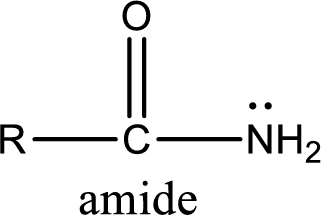
The difference between amine and amide is that in amine, the nitrogen atom is bonded to a hydrocarbon chain. In case of amides, the nitrogen atom is bonded to a carbonyl group.
(c)
Interpretation:
The given amine has to be classified as primary, secondary, or tertiary amine.
Concept Introduction:
Amine is an organic derivative. If in ammonia one or more alkyl, cycloalkyl, or aryl groups are substituted instead of hydrogen atom then it is known as amine. Depending on the number of substitution the amines are classified as primary, secondary or tertiary amine. Primary amine is the one in which only one hydrogen atom in ammonia is replaced by a hydrocarbon group. Secondary amine is the one in which only two hydrogen atoms in ammonia is replaced by a hydrocarbon group. Tertiary amine is the one in which all three hydrogen atoms in ammonia is replaced by a hydrocarbon group. The generalized structural formula for all the amines is,
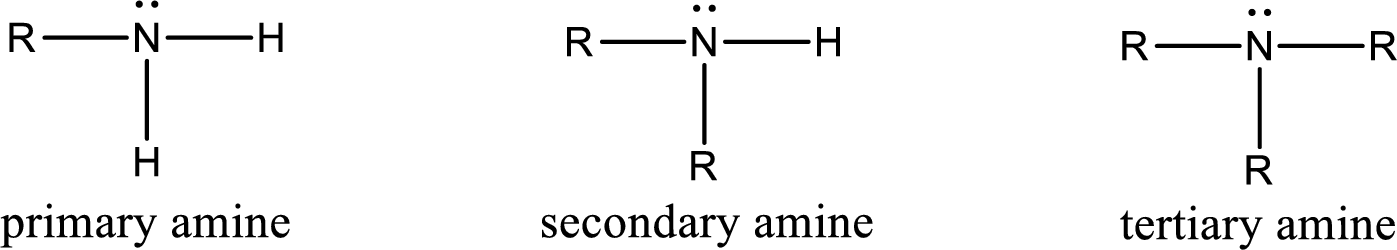
Amides are also organic derivative. In an amide, the nitrogen atom is bonded to a carbonyl group. The general structural formula of amide can be given as shown below,
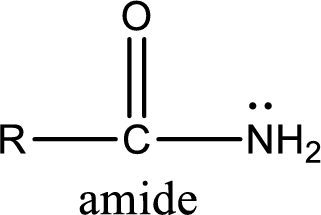
The difference between amine and amide is that in amine, the nitrogen atom is bonded to a hydrocarbon chain. In case of amides, the nitrogen atom is bonded to a carbonyl group.
(d)
Interpretation:
The given amine has to be classified as primary, secondary, or tertiary amine.
Concept Introduction:
Amine is an organic derivative. If in ammonia one or more alkyl, cycloalkyl, or aryl groups are substituted instead of hydrogen atom then it is known as amine. Depending on the number of substitution the amines are classified as primary, secondary or tertiary amine. Primary amine is the one in which only one hydrogen atom in ammonia is replaced by a hydrocarbon group. Secondary amine is the one in which only two hydrogen atoms in ammonia is replaced by a hydrocarbon group. Tertiary amine is the one in which all three hydrogen atoms in ammonia is replaced by a hydrocarbon group. The generalized structural formula for all the amines is,
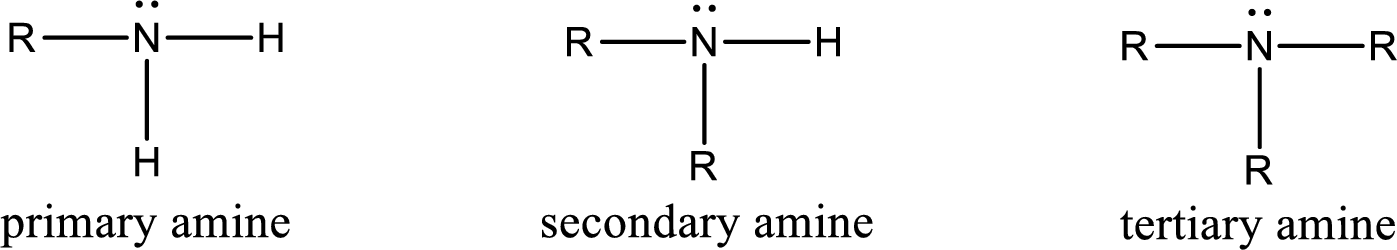
Amides are also organic derivative. In an amide, the nitrogen atom is bonded to a carbonyl group. The general structural formula of amide can be given as shown below,
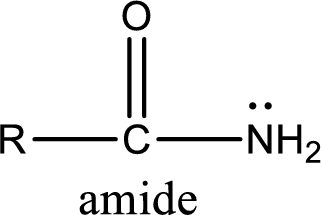
The difference between amine and amide is that in amine, the nitrogen atom is bonded to a hydrocarbon chain. In case of amides, the nitrogen atom is bonded to a carbonyl group.
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
Bundle: General, Organic, and Biological Chemistry, 7th + OWLv2 Quick Prep for General Chemistry, 4 terms (24 months) Printed Access Card
- For the reaction: CO2(g) + H2(g) --> CO (g) + H2O (g) Kc= 0.64 at 900 degrees celcius. if initially you start with 1.00 atmoshpere of carbon dioxide and 1 atmoshpere of hydrogen gas, what are the equilibrium partial pressuses of all species.arrow_forwardCan I please get this answered? With the correct number of significant digits.arrow_forwardDraw the Hofmann product of the dehydroiodination of this alkyl iodide. ☐ : + Explanation Check esc F1 2 3 I 88 % 5 F5 I. X © tBuOK Click and drag to sta drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Te BI BB F6 W E R Y S H Karrow_forward
- Can I please get help with this graph, if you could show exactly where it needs to pass through please.arrow_forwardDraw the condensed structure of 1,3-dihydroxy-2-pentanone. Explanation Check Click anywhere to draw the first atom of your structure. Х C © 2025 McGraw Hill LLC. All Rights Reserved. Terms of use +arrow_forward0.500 moles of NOCl are placed into a 1.00 L vessesl at 700K and after the system comes to equilibrium, the consentration of NOCl is 0.440 M. Calculate the equilibrium constant Kc for the reaction: 2NOCL (g) --> 2NO (g) + Cl2 (g)arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,



