
THERMODYNAMICS (LL)-W/ACCESS >CUSTOM<
9th Edition
ISBN: 9781266657610
Author: CENGEL
Publisher: MCG CUSTOM
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16.6, Problem 77P
An ammonia–water absorption refrigeration unit operates its absorber at 0°C and its generator at 46°C. The vapor mixture in the generator and absorber is to have an ammonia mole fraction of 96 percent. Assuming ideal behavior, determine the operating pressure in the (a) generator and (b) absorber. Also determine the mole fraction of the ammonia in the (c) strong liquid mixture being pumped from the absorber and the (d) weak liquid solution being drained from the generator. The saturation pressure of ammonia at 0°C is 430.6 kPa, and at 46°C it is 1830.2 kPa.
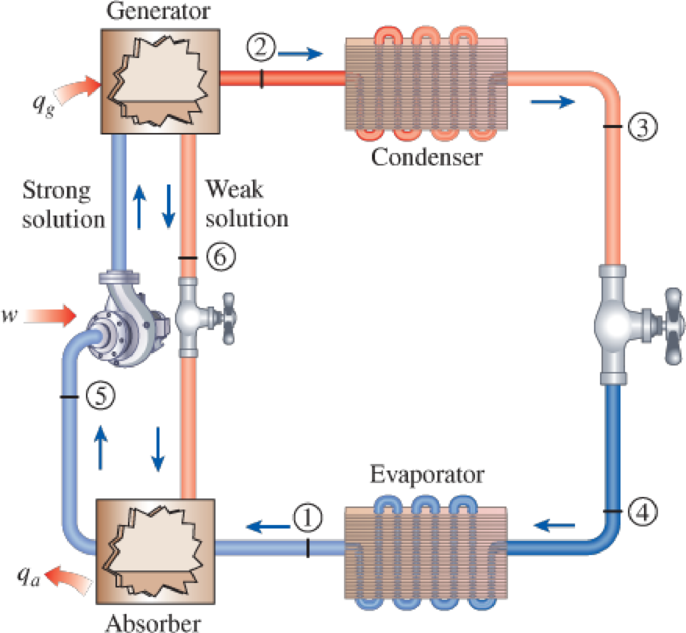
FIGURE P16–77
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
PLEASE SOLVE STEP BY STEP WITHOUT ARTIFICIAL INTELLIGENCE OR CHATGPT
SOLVE BY HAND STEP BY STEP
PLEASE SOLVE STEP BY STEP WITHOUT ARTIFICIAL INTELLIGENCE OR CHATGPT
SOLVE BY HAND STEP BY STEP
PLEASE SOLVE STEP BY STEP WITHOUT ARTIFICIAL INTELLIGENCE OR CHATGPT
SOLVE BY HAND STEP BY STEP
Chapter 16 Solutions
THERMODYNAMICS (LL)-W/ACCESS >CUSTOM<
Ch. 16.6 - Why is the criterion for chemical equilibrium...Ch. 16.6 - Write three different KPrelations for reacting...Ch. 16.6 - Is a wooden table in chemical equilibrium with the...Ch. 16.6 - A reaction chamber contains a mixture of CO2, CO,...Ch. 16.6 - A reaction chamber contains a mixture of N2and N...Ch. 16.6 - A reaction chamber contains a mixture of CO2, CO,...Ch. 16.6 - Which element is more likely to dissociate into...Ch. 16.6 - Prob. 8PCh. 16.6 - Prob. 9PCh. 16.6 - Prob. 10P
Ch. 16.6 - Prob. 11PCh. 16.6 - Prob. 12PCh. 16.6 - Prob. 13PCh. 16.6 - Prob. 14PCh. 16.6 - Prob. 15PCh. 16.6 - Prob. 16PCh. 16.6 - Prob. 17PCh. 16.6 - Prob. 18PCh. 16.6 - Prob. 19PCh. 16.6 - Prob. 20PCh. 16.6 - Prob. 21PCh. 16.6 - Prob. 22PCh. 16.6 - Prob. 23PCh. 16.6 - Determine the equilibrium constant KP for the...Ch. 16.6 - Prob. 26PCh. 16.6 - Prob. 27PCh. 16.6 - Carbon monoxide is burned with 100 percent excess...Ch. 16.6 - Prob. 30PCh. 16.6 - Prob. 31PCh. 16.6 - Estimate KP for the following equilibrium reaction...Ch. 16.6 - Prob. 33PCh. 16.6 - A mixture of 3 mol of N2, 1 mol of O2, and 0.1 mol...Ch. 16.6 - Prob. 35PCh. 16.6 - Prob. 36PCh. 16.6 - Prob. 37PCh. 16.6 - Prob. 38PCh. 16.6 - Prob. 40PCh. 16.6 - What is the equilibrium criterion for systems that...Ch. 16.6 - Prob. 43PCh. 16.6 - Prob. 44PCh. 16.6 - Prob. 45PCh. 16.6 - Prob. 47PCh. 16.6 - Prob. 48PCh. 16.6 - Prob. 51PCh. 16.6 - Prob. 52PCh. 16.6 - Prob. 53PCh. 16.6 - Prob. 54PCh. 16.6 - Prob. 55PCh. 16.6 - Prob. 56PCh. 16.6 - Prob. 58PCh. 16.6 - Prob. 59PCh. 16.6 - Prob. 60PCh. 16.6 - Prob. 61PCh. 16.6 - Using the Henrys constant data for a gas dissolved...Ch. 16.6 - Prob. 63PCh. 16.6 - Prob. 64PCh. 16.6 - Prob. 65PCh. 16.6 - Prob. 66PCh. 16.6 - A liquid-vapor mixture of refrigerant-134a is at...Ch. 16.6 - Prob. 68PCh. 16.6 - Prob. 69PCh. 16.6 - An oxygennitrogen mixture consists of 30 kg of...Ch. 16.6 - Prob. 71PCh. 16.6 - Prob. 72PCh. 16.6 - Prob. 73PCh. 16.6 - Prob. 74PCh. 16.6 - Prob. 75PCh. 16.6 - Prob. 76PCh. 16.6 - An ammoniawater absorption refrigeration unit...Ch. 16.6 - Prob. 78PCh. 16.6 - Prob. 79PCh. 16.6 - Prob. 80PCh. 16.6 - One lbmol of refrigerant-134a is mixed with 1...Ch. 16.6 - Prob. 82RPCh. 16.6 - Prob. 83RPCh. 16.6 - Prob. 84RPCh. 16.6 - Prob. 85RPCh. 16.6 - Prob. 88RPCh. 16.6 - Prob. 89RPCh. 16.6 - Prob. 90RPCh. 16.6 - Prob. 91RPCh. 16.6 - Prob. 92RPCh. 16.6 - A constant-volume tank contains a mixture of 1 mol...Ch. 16.6 - Prob. 94RPCh. 16.6 - Prob. 95RPCh. 16.6 - Prob. 96RPCh. 16.6 - Prob. 97RPCh. 16.6 - Prob. 99RPCh. 16.6 - Consider a glass of water in a room at 25C and 100...Ch. 16.6 - Prob. 101RPCh. 16.6 - Prob. 102RPCh. 16.6 - Prob. 105RPCh. 16.6 - Prob. 106RPCh. 16.6 - Prob. 107RPCh. 16.6 - Prob. 108RPCh. 16.6 - Prob. 109FEPCh. 16.6 - Prob. 110FEPCh. 16.6 - Prob. 111FEPCh. 16.6 - Prob. 112FEPCh. 16.6 - Prob. 113FEPCh. 16.6 - Prob. 114FEPCh. 16.6 - Propane C3H8 is burned with air, and the...Ch. 16.6 - Prob. 116FEPCh. 16.6 - Prob. 117FEPCh. 16.6 - The solubility of nitrogen gas in rubber at 25C is...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Consider the bar, shown in Figure 1 that undergoes axial displacement due to both a distributed load and a point force. The bar is of cross-sectional area A = 1.10-3 m², and has a modulus of elasticity E = 100 GPa. 1(x) = 5 kN/m x=0.0 x=2.0 2.0m 10 kN Figure 1: Bar domain with varying distributed forces. a) The general form of the governing equations describing the bar's displacement, u(x), is given by, d (AE du(x)) -) +1(x) = 0. d.x dx What are the accompanying boundary conditions for this bar? b) Using the mesh in Figure 2, form the basis functions associated with element 2 and write the FEM approximation over the element. 1 2 3 1 2 1m 1m Figure 2: Mesh of 2 elements. Elements are numbered with underlines. c) The general form of the element stiffness matrix system, with nodes indexed by i and j, is, AE Uj N;(x)l(x)dx – Ng(0)f(0) ¥ [4]}]{{}}={{{}\(\\+} + {N(2)f(2) = N (0)5() }, (1) 0, respectively. L = (2) where f(2) and f(0) denote the boundary forces at positions x 2 and x Evaluate…arrow_forwardanswer pleasearrow_forwardamination) Question 1 Consider the bar, shown in Figure 1, that undergoes axial displacement due to both a distributed load and a point force. The bar is of cross-sectional area A = 1.103 m2, and has a modulus of elasticity E = 100 GPa. 1(x) = 5 kN/m 10 kN X x=0.0 x=2.0 2.0m Figure 1: Bar domain with varying distributed forces. a) The general form of the governing equations describing the bar's displacement, u(x), is given by, d (AE du(x)) + 1(x) = 0. dx dx What are the accompanying boundary conditions for this bar? MacBook Air a 会 DII F5 F6 F7 F8 80 F3 F4 0/ 20 [8 marksl 8 FOarrow_forward
- show workingarrow_forwardCFD help Figure 3: Advection equation, solution for three different timesteps. Q1) Provide an explanation what conditions and numerical setup could explain the curves. Identify which of the three curves is the first, second and third timestep.arrow_forwardanswer pleasearrow_forward
- Figure 3 shows the numerical solution of the advection equation for a scalar u along x at three consecutive timesteps. 1.0 0.8- 0.6 0.4- 0.2 0.0 00 -0.2 -0.4 -0.6- 3.0 3.5 4.0 4.5 5.0 5.5 6.0 6.5 Figure 3: Advection equation, solution for three different timesteps.arrow_forwardQuestion 2 Figure 3 shows the numerical solution of the advection equation for a scalar u along x at three consecutive timesteps. 1.0 0.8- 0.6- 0.4- 0.2- 0.0- -0.2- -0.4- -0.6 3.0 3.5 4.0 4.5 5.0 5.5 6.0 6.5 Figure 3: Advection equation, solution for three different timesteps. a) Provide an explanation what conditions and numerical setup could explain the curves. Identify which of the three curves is the first, second and third timestep. b) Consider explicit schemes with central and upwind discretisations. Explain how each of these candidate discretisations could produce the behaviour shown in Figure 3. c) Determine the CFL number that was used in the simulation for each of the candidate schemes for all possible updates. Assume that the timestep and mesh-width used are constant. Read the data to two digits of accuracy from Figure 4 shown at the end of the question, which is an enlarged version of Figure 3. Demonstrate your method and input data for one calculation, but then use a…arrow_forwardanswer pleasearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning
Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning

Refrigeration and Air Conditioning Technology (Mi...
Mechanical Engineering
ISBN:9781305578296
Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:Cengage Learning
Chemical and Phase Equilibrium; Author: LearnChemE;https://www.youtube.com/watch?v=SWhZkU7e8yw;License: Standard Youtube License