
Concept explainers
Interpretation: To identify the correct conjugate acid-base pair.
Concept introduction: Whenever an acid donates a proton, it changes into a base, and whenever a base accepts a proton, an acid is formed. Conjugate acid-base pair can be defined as an acid and a base which differ only by the absence or presence of a proton.
Answer to Problem 2RQ
a, e, and f represents correct conjugate acid-base pair.
Explanation of Solution
Reason for correct option:
(a)
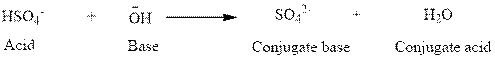
Here,
(e)

Here,
(f)

Here,
Reason for incorrect options:
b)

Here,

Here,
c)


Here,
d)

Here,

Here,
Chapter 16 Solutions
World of Chemistry
- For each scenario below, select the color of the solution using the indicator thymol blue during the titration. When you first add indicator to your Na2CO3solution, the solution is basic (pH ~10), and the color is ["", "", "", "", ""] . At the equivalence point for the titration, the moles of added HCl are equal to the moles of Na2CO3. One drop (or less!) past this is called the endpoint. The added HCl begins to titrate the thymol blue indicator itself. At the endpoint, the indicator color is ["", "", "", "", ""] . When you weren't paying attention and added too much HCl (~12 mL extra), the color is ["", "", "", "", ""] . When you really weren't paying attention and reached the second equivalence point of Na2CO3, the color isarrow_forwardThe following reaction is run in which the initial conditions include only methane (CH4) at a concentration of0.115 M. Once equilibrium was established, the concentration of acetylene (C2H2) was measured to be 0.035M. What is the value of the equilibrium constant, K?2 CH4 (g) ⇋ C2H2 (g) + 3 H2 (g)arrow_forwardCalculate the equilibrium concentration of carbon dioxide for the following reaction:2 COF2 (g) ⇋ CF4 (g) + CO2 (g) Kc = 2.00 at 10.00 °C. at equilibrium [COF2] = 0.255M; [CF4] = 0.118Marrow_forward
- In a benzene derivative that has -CH2CH3, indicate how it can be substituted by -COOH.arrow_forwardIn a sulfonated derivative of benzene, indicate how -SO3H can be eliminated.arrow_forwardWhat is the equilibrium expression (law of mass action) for the following reaction:CO2 (g) + H2O (l) ⇋ H+ (aq) + HCO3- (aq)arrow_forward
- Indicate the compound resulting from adding NaOH cyclopentane-CH2-CHO.arrow_forwardUse the provided information to calculate Kc for the following reaction at 550 °C: H2(g) + CO2(g) ⇌ CO(g) + H2O(g) Kc = ?CoO(s) + CO(g) ⇌ Co(s) + CO2(g) Kc1 = 490CoO(s) + H2(g) ⇌ Co(s) + H2O(g) Kc2 = 67arrow_forwardCalculate Kc for the reaction: I2 (g) ⇋ 2 I (g) Kp = 6.26 x 10-22 at 298Karrow_forward
- For each scenario below, select the color of the solution using the indicator thymol blue during the titration. When you first add indicator to your Na2CO3solution, the solution is basic (pH ~10), and the color is ["", "", "", "", ""] . At the equivalence point for the titration, the moles of added HCl are equal to the moles of Na2CO3. One drop (or less!) past this is called the endpoint. The added HCl begins to titrate the thymol blue indicator itself. At the endpoint, the indicator color is ["", "", "", "", ""] . When you weren't paying attention and added too much HCl (~12 mL extra), the color is ["", "", "", "", ""] . When you really weren't paying attention and reached the second equivalence point of Na2CO3, the color isarrow_forwardTo convert cyclopentane-CH2-CHO to cyclopentane-CH2-CH3, compound A is added, followed by (CH3)3CO-K+, DMS at 100oC. Indicate which compound A is.arrow_forwardIndicate how to obtain the compound 2-Hydroxy-2-phenylacetonitrile from phenylmethanol.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





